1. What is electrowinning?
Electrowinning is a process in which metal ions present in an electrically conductive solution are separated using a direct current. This is achieved when a direct current is applied across an anode and cathode that are submerged in an electrically conductive solution causing the metal to deposit on the cathode. We say that metals like these were electrowon.
FREE Download - Copper Recovery Brochure
To download fill the form below
2. What are electrically conductive solutions?
Electrically conductive solutions are solutions that are formed when an electrolyte is dissolved in a polar solvent, for example water. When the electrolyte is put into a polar solvent, it is dissolved into negatively charged anions and positively charged cations. When a direct current is applied to the solution, the anions are drawn to the positively charged electrode (or anode) and cations are drawn to the negatively charged electrode (or cathode). The clue is pretty much in the name; electrically conducting solutions are solutions capable of conducting electricity for manipulating the movement of the ions formed by dissolving the electrolyte.
3. What are the differences between electrowinning and electrorefining?
What is electrorefining?
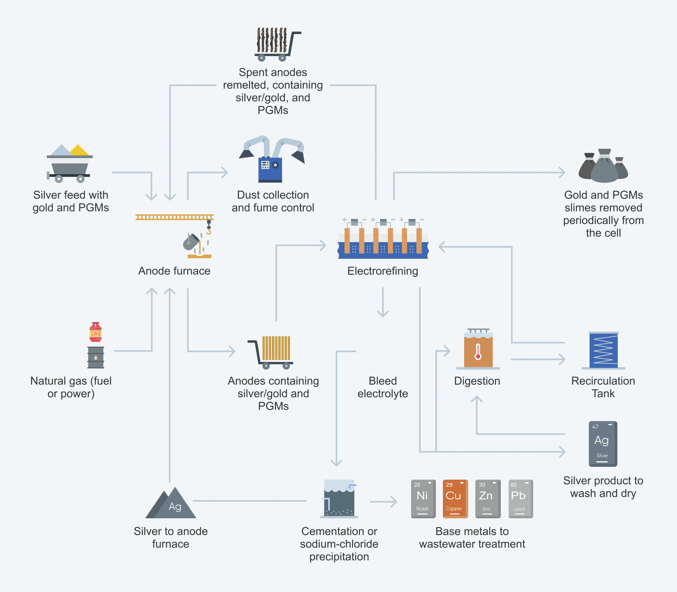
Electrolytic refining utilizes anodes comprised of impure metal. When the current is passed through the solution, the anode is corroded into the solution, causing the metal cation to migrate over to be re-plated onto the cathode.
In electrowinning, on the other hand, the metal is already dissolved into solution hence the anode is inert.
Conventional electrorefining process:
electrowinning process with emew:
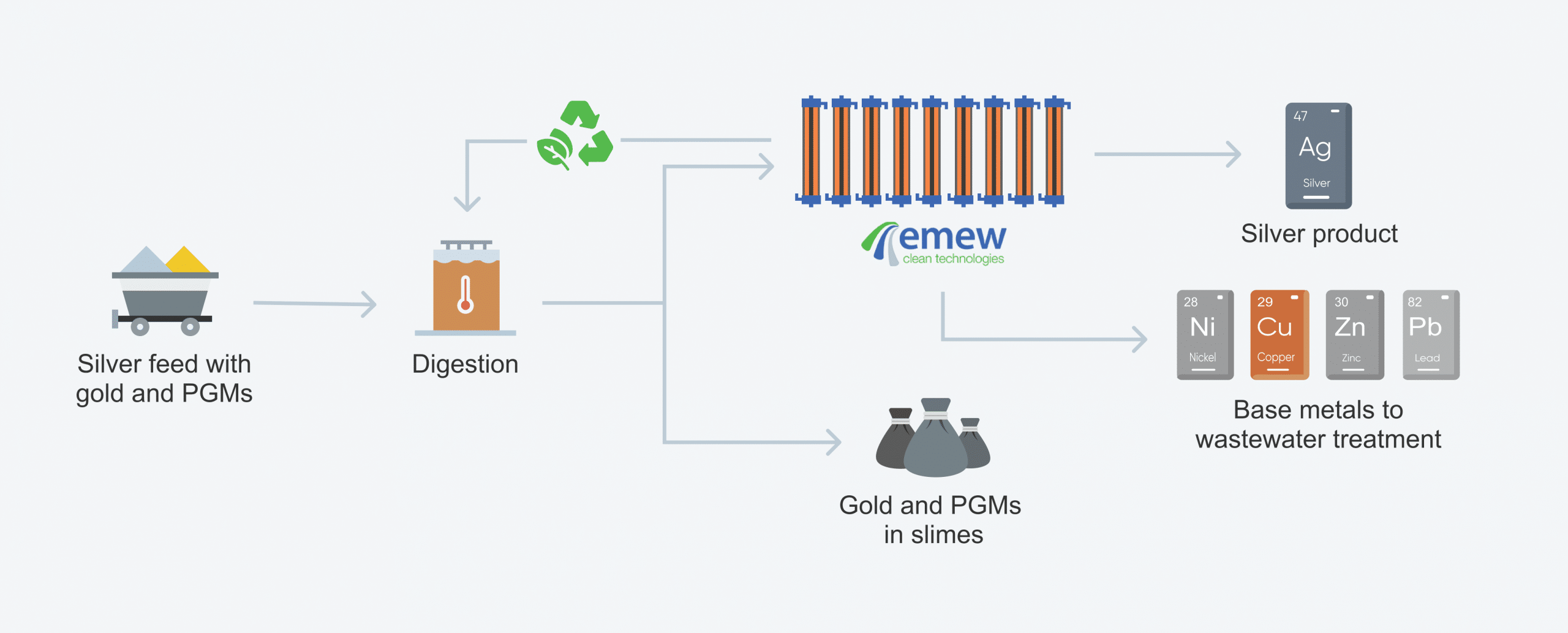
4. Why do we need electrorefining and electrowinning processes?
A lot of metals, especially non-ferrous metals, are hard to find in nature in their purest form. Usually, these metals are found in their mineralized forms, so they need to be reduced to their metallic forms for further usage. Electrowinning is both cost and energy-efficient way of doing this. This is why these are commonly used processes for pure metal production and recovery.
5. What are the most commonly electrowon metals?
The most commonly electrowon metals are copper, gold, silver, zinc, cobalt, and nickel. Electrowinning as an extraction process is especially important for copper and silver. And with advanced “vortex” emew electrowinning technology the process is even more efficient ensuring the purity of metal can be plated to as high "Five Nines" (99.999%) along with the ability to deplete metals down to low concentrations (< 10 ppm).

6. Where can electrowinning processes be used aside from mining?
Electrowinning processes can also be used for wastewater treatment and general recycling. The principle is still the same, any impure material can be leached into a conducting solution, then the DC voltage is applied through the solution and the desired material can be extracted (of course, there are certain limitations such as metal concentration, temperature, acidity levels, chemical components, and more). When it comes to wastewater treatment, electrowinning is a win-win situation. Less soluble metals make their way to landfill along with the recovery of valuable metals which can be sold to offset the processing cost.
7. How is electrowinning used for recycling and why?
Electrowinning can be used as a processing step for the recycling of non-ferrous metals. Non-ferrous metals can be recycled infinitely without losing any of their properties. This feature makes electrowinning an ideal process to recover pure metals from complex, mixed metal feeds. In many cases, it is cheaper to recycle metals instead of mining ore, processing it and extracting pure metals. And this is especially true considering continuously decreasing metal grades in existing mines and deposits.

8. Which process is more frequently used, electrowinning or electrolytic refining?
Deciding on what process to opt for will depend on the situation. Electrorefining is used when an anode is cast from impure metal. Electrowinning is employed when the metal is already dissolved into solution, or when it is more feasible to employ a hydrometallurgical digestion step as opposed to casting impure anodes. Electrowinning can also be done on a smaller scale and for more niche applications. Both electrowinning and electrolytic refining can produce very high purity metals exceeding 99.99%.
9. What are the issues of conventional electrowinning?
For the most part, electrowinning is a rather straightforward and simple process. As the process advances, the concentration of the target metal is depleted from the solution as the metal is plated onto the cathode. As the concentration of metal decreases, so-called depletion zones start to form next to the cathode. These are the zones where the concentration of the targeted metal is lower than in the bulk solution. This can be problematic as if there are other metal ions present in the solution they will start to plate onto the electrode resulting in impurities and low current efficiency.
10. Is it possible to improve the electrowinning process?
Luckily advances in electrowinning have overcome these problems. Vortex emew electrowinning technology offers an alternative approach to conventional electrowinning ensuring much better results. The emew electrowinning technology uses cylindrical cells in which the electrolyte is rapidly circulated past the electrodes at very high velocities which overcomes the issues associated with depletion zones, and enables depletion of metal to much lower concentrations while still achieving the highest purity of metal possible. On top of that, emew electrowinning cells are closed systems that completely eliminate acid mist and other noxious gasses making it a much safer place to work.

References:

References:
http://www.ct.ufrgs.br/ntcm/graduacao/ENG06631/5-b_copper.pdf







