A common question we get here at emewCorporation is: “What is the power consumption of emew electrowinning technology”. This is a very important question because power consumption is one of most significant costs involved in electrowinning, regardless of whether it is conventional or emew.
Power consumption varies depending on the application and the metal being recovered. Let’s take a look at the two key factors affecting power consumption, and what that means for your electrowinning application:

The dc power consumption in any electrowinning cell is dependent on two factors: the cell voltage and the current efficiency.
The first factor we’re going to look at is cell voltage which is governed by several factors:
- The conductivity of the solution. The cell voltage will be higher for solutions with low conductivity, which in turn affects the power consumption. In low pH solutions, acid is produced as the target metal is electrowon from solution. Hydrogen ions characteristic of acidic solutions are very conductive and have the effect of lowering cell voltage.
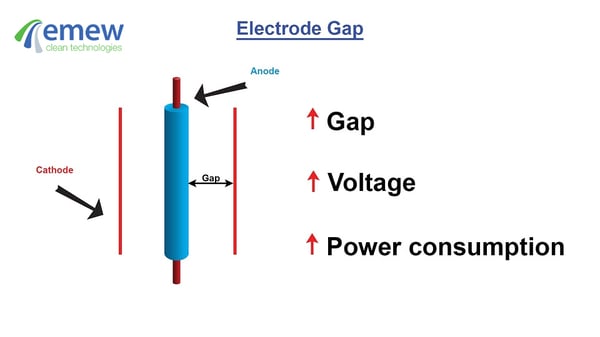
- The electrode gap between the anode and the cathode. The larger the gap, the higher the cell voltage. In some countries with very high power costs such as Chile, the anode and cathode gap can be decreased to reduce power consumption. Furthermore, as metal is plated onto the cathode over time between harvesting cycles, the electrode gap decreases thus reducing cell voltage.
- The temperature of the solution. Increasing the temperature of the electrolyte can be used to lower the cell voltage.
- The higher the current density the higher the cell voltage so there is a “power penalty” for running at a higher production rate. Typically the electrolyte rises in temperature with the higher current density and this offsets the increase in cell voltage.
The second factor, current efficiency, is the ratio of actual metal plated from an electrolyte by applying a current compared to the theoretical mass predicted by Faraday’s law.

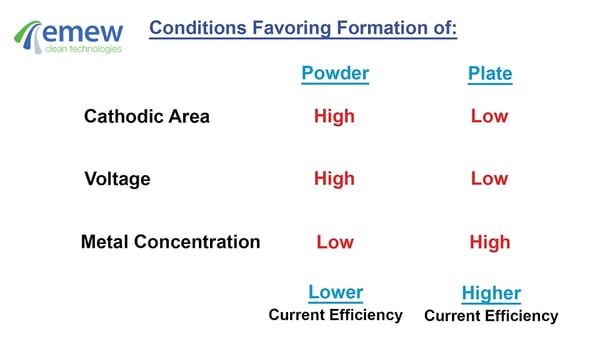
The cathodic area, voltage and metal concentration can adversely affect current efficiency. Large cathodic area, high voltage and low metal concentration generally lead to lower current efficiency. In general, these conditions favour the production of metal powders with lower current efficiency. Metal plate on the other hand is generally the result of high current efficiency. Additives such as glues can also be used to enhance current efficiency in some applications.
Another consideration is that you need to supply alternating current or AC power to the rectifier and the conversion to direct current or DC power is not 100% efficient. For most applications, emewCorporation supplies high efficiency switch mode rectifiers to ensure the highest conversion efficiency over a range of turndown.
Pumps are used to circulate electrolyte from the feed tank through the emew cells and also consume power. The pumping requirements must also be considered when looking at the overall power requirements.
So when we get the question, “What is the power consumption”? A common response is, “It depends” and you can see why since there are many factors that affect power consumption. We can provide estimates based on our experience, however actual numbers can only be determined from empirical data. We offer lab cell test programs to confirm process and operating data using actual process electrolyte to assist in the economic evaluation of your metal recovery project.
Find out more at www.emew.com. And don’t forget to follow us on Twitter, LinkedIn, and YouTube and Like Us on Facebook.
That’s all for today and be sure to tune in again next time.







