Acid mine drainage: we’ve all heard of it, and its negative reputation. But what if we told you that it’s not all bad? What if this environmental pollutant was recovered and transformed into a profit-turning resource? [Bejan & Bunce, 2015] There are varying methods of dealing with acid mine drainage (AMD), but only some of them are economically viable and effective long-term. This blog post is going to dive into some of the solutions for the treatment of AMD, and how emew technology provides one of the most promising alternatives, specifically for copper recovery from acid mine drainage.
Copper Recovery from an Acid Mine Drainage Stream
Can I recover copper from this? Copper recovery from the ground up
A question that we hear from Clients every day is "Can I recover (copper - or other metals) from this?" The material in question can be concentrated wastes, dilute effluents,
Topics: effluent treatment, environmental, copper, Wastewater Treatment, metal recycling, mining, Refinery Optimization, copper recovery, copper recycling
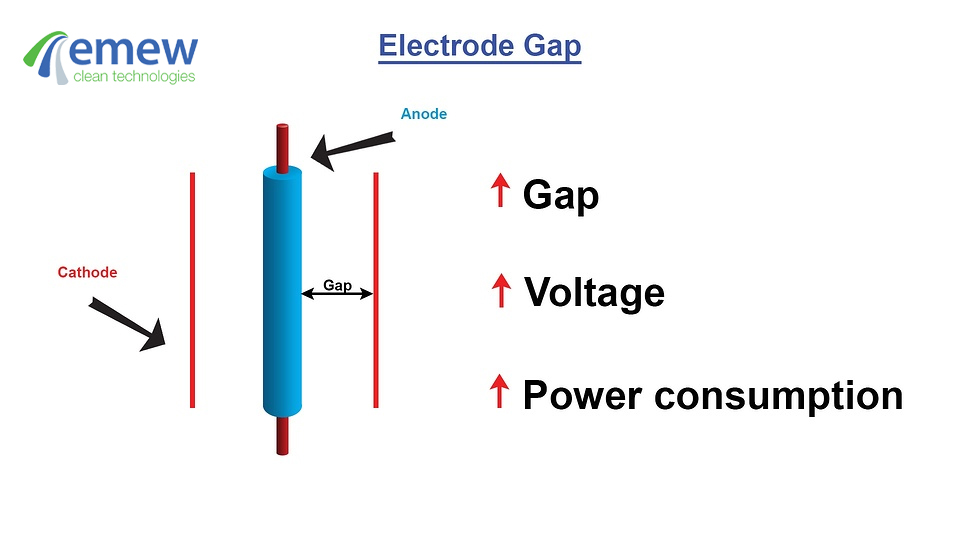
The electrowinning of copper is an electrolytic process that uses electricity to recover dissolved copper from solution as
Cu2+(aq) + 2e- -->Cu(s) (E0 = +0.34V)
Copper easily dissolves in acids including sulphuric, nitric and hydrochloric. Recovering copper cathode from acidic
Topics: copper, electrowinning, emew, Refinery Optimization
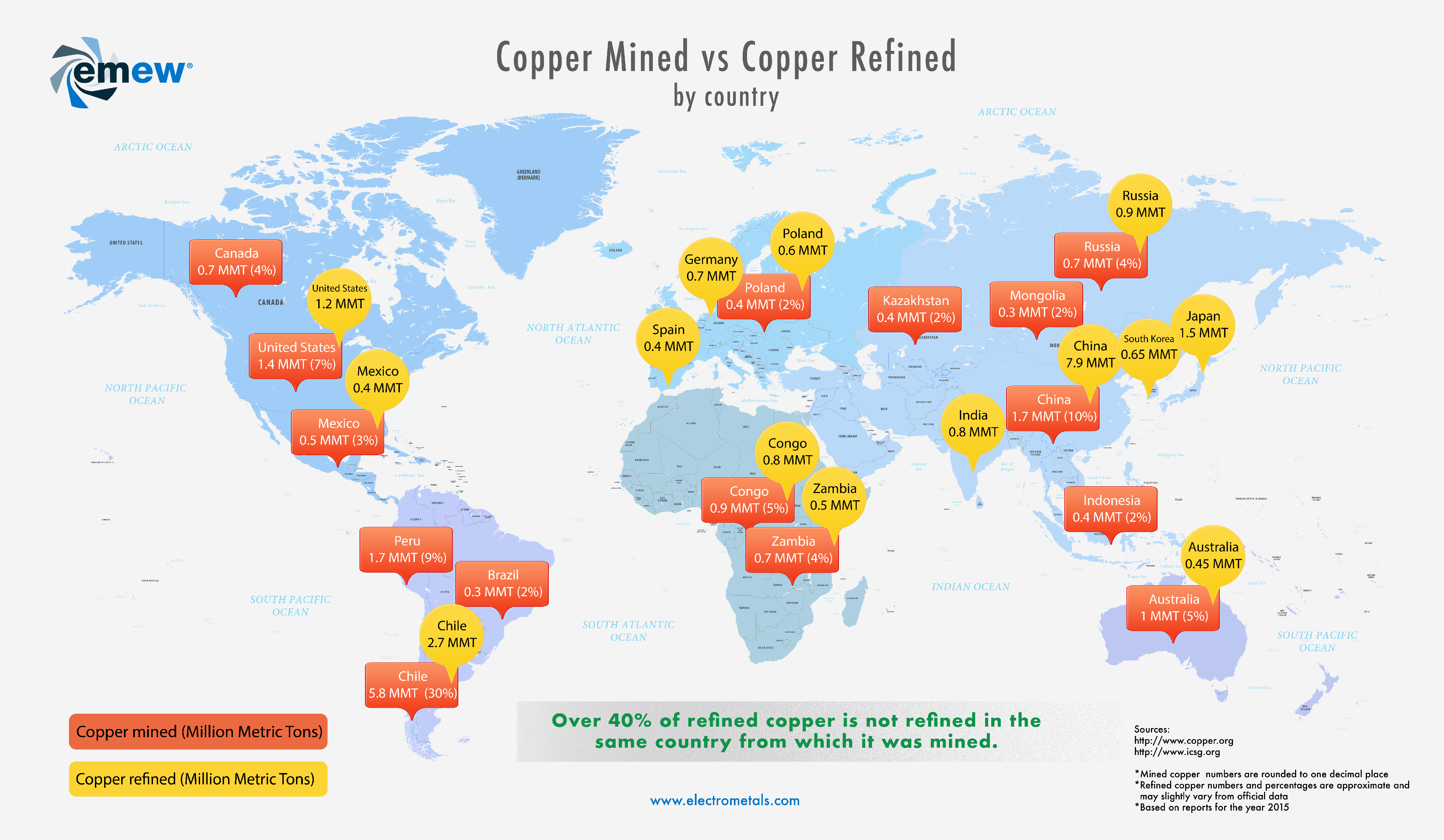
Where is copper mined and refined?
Taking a closer look at worldwide copper production statistics we found that some of the biggest copper-producing countries do not necessarily refine the copper at home. The reasons for this depend on various factors. We wanted to find out how much of copper is not actually refined in the same countries from which it was mined.
Topics: copper, Small Mine Production
Lab tests such as this are an ideal way to demonstrate the recovery capabilities of emew for a variety of electrolytes and feed materials. In most cases, these tests can be done right at your site so you can see the results for yourself.
This particular test is to demonstrate the recovery of copper cathode from a copper sulphate feed solution. We can test a range of feed materials to recover not only copper, but also silver, nickel, tin and others. This copper sulphate solution was prepared ahead of time and roughly 3L transferred to the feed tank.
Topics: copper, electrowinning, emew, General
What pops into your mind when you see the word, "liberation"?
---
The word liberation comes from the latin word, liberatus, which literally means "to set free" or "to deliver", and can easily apply to myriad of different topics from politics and religion to science and industry.
Particularly in the copper industry, the word liberation applies to the act of freeing copper specifically from refinery bleed electrolyte. The process that starts with mining and carries on through smelting and electrorefining is complex with many process operations. Let's take a closer look at the story of 'liberating copper'.
On average, mined ore contains about 1% copper and in order to recover this copper from the rock it requires several physical and chemical processing and refining steps in order to produce market ready copper metal.
Topics: copper, nickel, Refining, Refinery Optimization