Way back in elementary school when you thought of nickel, the first image that popped into your head was probably that nice shiny 5¢ coin. Even though we hardly carry change purses anymore, nickel is still ever present in our day-to-day lives. From the shiny stainless steel trim on your
The truth is, high-grade nickel reserves are being readily depleted, and mining companies are looking for new ways to recover nickel, this highly desired base metal. I’d like to dig a little deeper into the recovery of nickel, specifically from low-grade nickel ores on the Earth, and from bleed solutions from the electrorefining of other metals.
The Importance of Nickel Recovery
Nickel is the fifth most abundant element on Earth, at an average bulk of 2.7%. However, the average nickel content of the crust is estimated at 0.008 percent. With such low content, it makes it obvious why nickel mining is such an important industry, and why recovery of nickel and nickel recycling is becoming increasingly significant in our consumer-based society.
Nickel has many important properties that contribute to its widespread use in industry, these properties
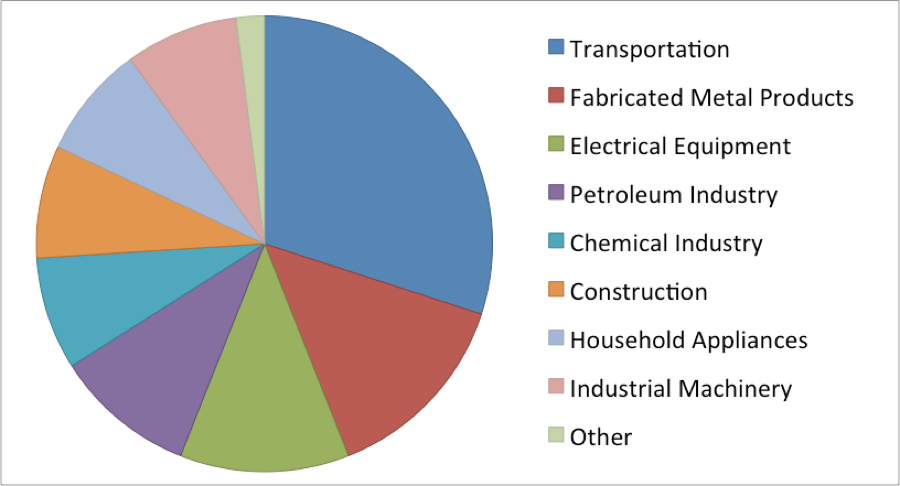
Fig. 1: Primary Nickel Consumption in the US in 2012
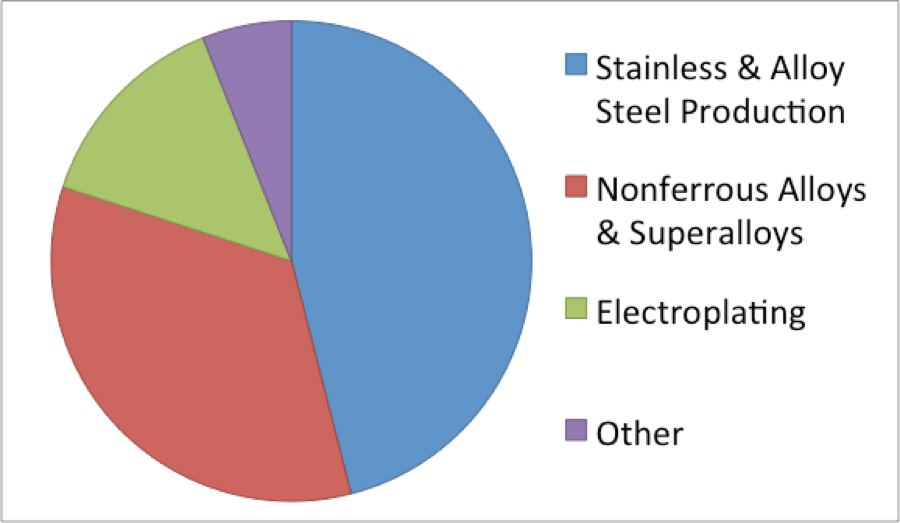
Fig. 2: End Use Nickel Consumption in the US in 2012
Worldwide, approximately two million tonnes of nickel are used each year: 2/3 from primary sources and 1/3 from the recycling of nickel from end-of-use scrap. In the US it was reported that 99 000 tons of nickel were recovered from scrap in 2011, up from the 63 500 tons of nickel recovered from scrap in 2009, indicating from recycling is on the rise.
Production of Nickel from Lateritic and Sulfide Ores
In 2009, the world’s top nickel producers were Russia, Indonesia, Canada, Australia, and New Caledonia. Nickel is produced primarily from laterite and magmatic sulfide deposits. Laterites comprise approximately 70% of the world’s nickel deposits, while only contributing to about 40% of global production. Laterite is rock formed in tropical and subtropical climates, over long periods of erosion, and nickel-bearing laterites have an average content of 1-1.6% Ni.
At present, sulfide nickel deposits are the primary source of mined nickel. Sulfide ore grades range from 0.15-8% Ni, with 93% of known deposits ranging from 0.2-2% Ni.
All nickel ores have relatively low nickel content, the classification is as follows: high-grade nickel ore has Ni content greater than 1.8%, middle-grade nickel ore has Ni content between 1.3-1.7%, low-grade nickel ore has Ni content between 0.6-1.2%. Nickel is continuously mined, even at these low percentages of ores, because it is in high demand.
The variation in nickel concentration depends on the type of rock deposit that is weathered - ie. peridotite, dunite, pyroxenite, or serpentinite. Laterite formed from the weathering of serpentinite, for example, contains 45-55% iron and about 1% nickel, this is known as nickeliferous iron laterite. The other type of nickeliferous laterite is known as nickel silicate, which contains less than 30% iron, 30% silicon dioxide, and about 1.6% nickel. In general, laterite has high concentrations of iron, titanium, and aluminum oxides.
With these low percentages, not all nickel recovery from laterite mining is economically feasible. In 2004, Dalvi, Bacon, and Osborne
Nickel Recovery and Techniques of Mining Nickel from Lateritic Deposits
I’d first like to focus on the processing techniques of mining nickel from lateritic deposits, which can be a challenge to execute efficiently. Lateric ores are frequently treated by pyrometallurgical processes - using a furnace to smelt the dried ore and carbon as a reducing agent. Sulfur can be added if a matte is required, and further refining can be used to produce ferro-nickel or matte.
Limonitic (oxide)
The method of PAL involves preheating the slurried
An excellent example the use of PAL on low-grade lateritic nickel ores is the Ambatovy mine in Madagascar, a joint venture between Sherritt, Sumitomo, and Korea Resources. This is one of the largest lateritic nickel mines in the
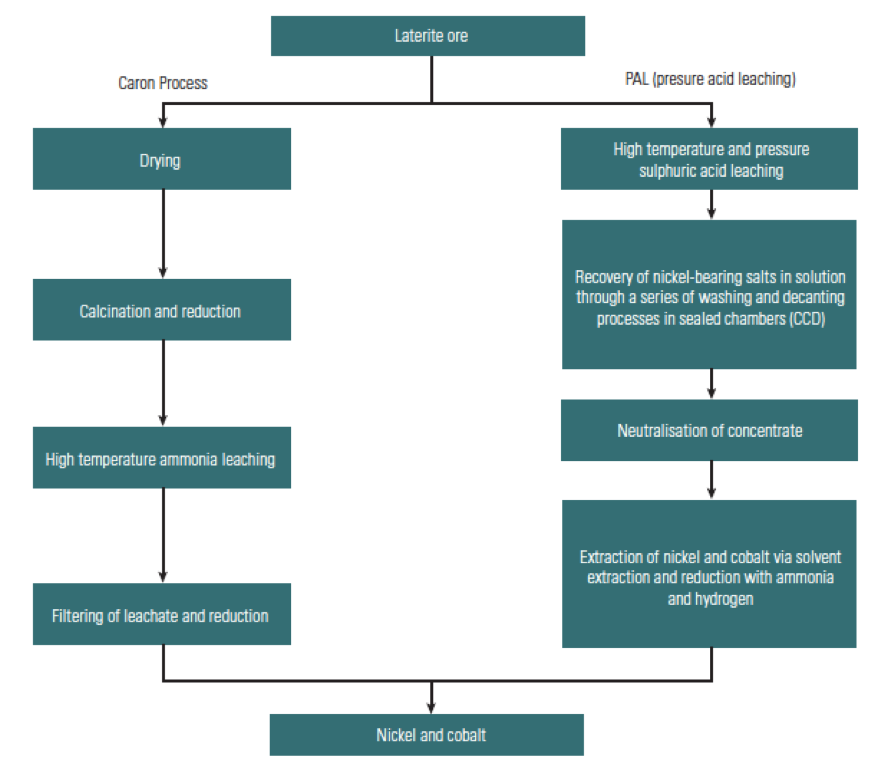
Fig. 3: Flow Sheet of the Processing of Lateritic Ores (Natural Environment Research Council, 2008)
Recovery of Nickel and Processing of Magmatic Sulfide Ores
The processing of magmatic sulfide ores differs from laterites: sulfide ores are crushed in multiple steps to separate the ore minerals from the gangue. At each stage the ore is separated by size, using vibrating screens, as well as magnetic separation of iron-rich pyrrhotite. After crushing, the ore is mixed with water to create a slurry and is ground to a powder. Water is then added again, to produce a suspension, and air is blown upwards through the tanks.
Here, chemical separation is used: chemicals are added to make some minerals repel water, allowing the minerals to float to the surface, this froth is then removed – designed to remove copper concentrate. The second stage of froth flotation produces a nickel concentrate of 10-20% Ni, along with other by-products and gangue.
Smelting is the next stage, designed to recover as much metal as possible. Flash smelting is a common method: dry concentrates are fed into a furnace and heated to a liquid matte and slag. From this step, sulfide matte can be recovered, which contains cobalt, and nickel (about 70%). Iron is recovered in the slag, and sulfur as sulfur dioxide. At this stage, pyrometallurgy and hydrometallurgy processes can be used to refine the metals. In pyrometallurgy, metals are separated from the contents of the matte by using heat – to separate based on chemical and physical characteristics such as melting point and density.
In hydrometallurgy, metals are separated based on
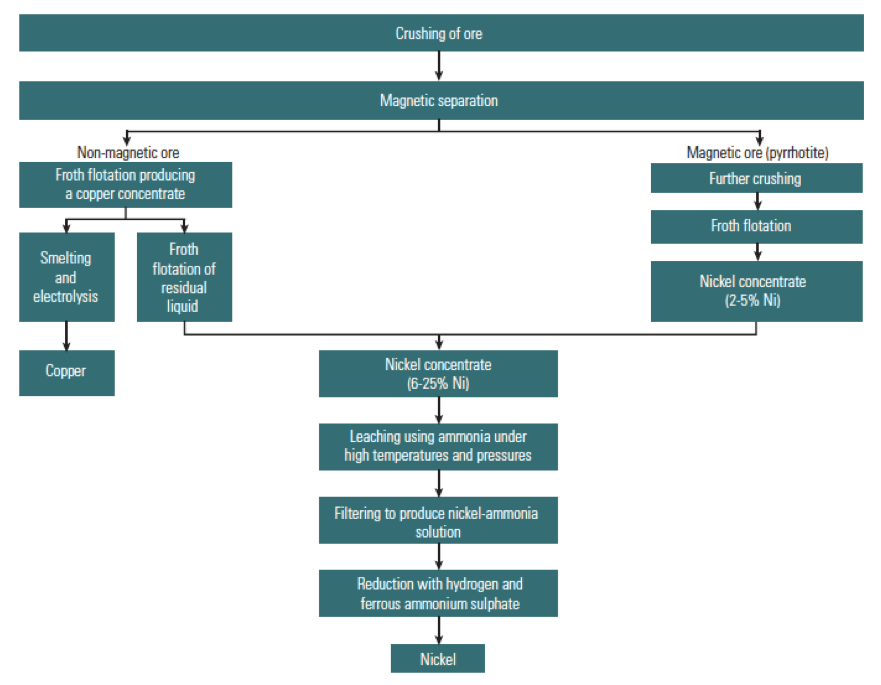
Fig. 4: Flow Sheet of the Processing of Magmatic Sulfide Ores (Natural Environment Research Council, 2008)
Enhanced Methods of Nickel Recovery
New processes are being developed in order to be more cost-effective and environmentally sound. One example of this is Activox®, which removes the smelting step, and uses ultra fine grinding and pressure oxidation of the concentrate in an autoclave, prior to solvent extraction and metal precipitation.
Bioleaching is another process that is being looked at for low-grade ores, ideal for reworking waste dumps. This method, under atmospheric pressure, is being used in the Talvivaara project in Finland, a sulfide mineral resource of only 0.27% Ni.
Low-grade ores, like nickel sulfide, are abundant on Earth. Nickel sulfide deposits contain high levels of magnesium silicate gangue minerals (MgO) and can be difficult to process with conventional flotation and smelting technologies. MgO is hydrophilic, which interferes with
High-grade nickel ores are almost
Whether high-grade or low-grade, nickel ores need to be processed after mining, in order to upgrade their nickel content from 1-4% Ni to 10-20%. Concentrating the nickel ores usually takes place close to the mine site, and involves chemical and physical processes to crush the ore and separate the nickel-bearing and gangue minerals.
Mining nickel directly is the obvious route to obtaining pure nickel, however, nickel can also be recovered as a byproduct of other metals. For example, copper electrolysis requires a bleed to eliminate the buildup of impurities that accumulate over time as a result of the electrorefining of impure copper anode to produce
Nickel can be recovered from the bleed stream of a copper
In most copper refinery electrolytes the concentration of copper is between 35-60 g/L Cu (typically between 40 and 50 g/L), 120-200 g/L H2SO4 (typically between 150 and 200 g/L), and 0.3-25 g/L Ni. Soluble anode impurities continuously dissolve into the electrolyte, which is why they must be continuously removed from the bleed stream to prevent build up. Nickel is not the only metal removed from bleed streams, so are arsenic, bismuth, cobalt, iron, antimony, and 1-2% of the copper (owing to the fact that the corrosion of copper at the anode is faster than the plating at the cathode).
Nickel Extraction from Bleed Streams with SX
Another method for extraction of nickel from the bleed stream is the use of solvent extraction (SX). Copper-selective solvents can be chosen, and many steps are followed to get nickel and copper powder from the bleed. The following block flow diagram was shown in a paper by A. Agrawal et al. in 2012, demonstrating the steps of SX for nickel and copper recovery, Figure 5.
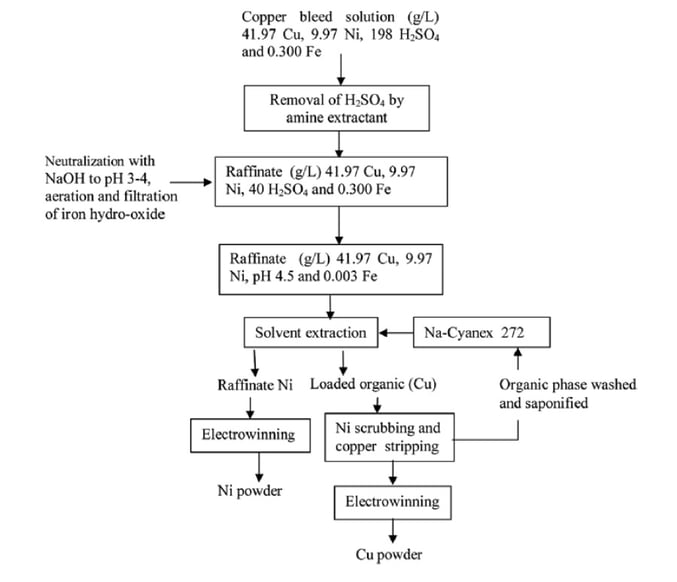
Fig. 5: Flow diagram for the treatment of copper bleed stream by solvent extraction route (A. Agrawal et al., 2012.)
SX is a tool used in the processing of complex and secondary
Ammoniacal solutions are used in
Sulfuric acid is the most common medium for hydrometallurgical metal extraction; SX processes often report the separation of nickel and cobalt from dilute sulfuric acid solution. This separation is carried out by cation-exchange type reagents. Ion exchange (IX) is used frequently in metal finishing industries for nickel recovery. Some copper refinery
Nickel and Copper Powder Production through Hydrogen Reduction
Another method of copper and nickel powder production, reported in 2008 by A. Agrawal et al., uses hydrogen reduction. This method takes the copper bleed and uses hydrogen gas to produce
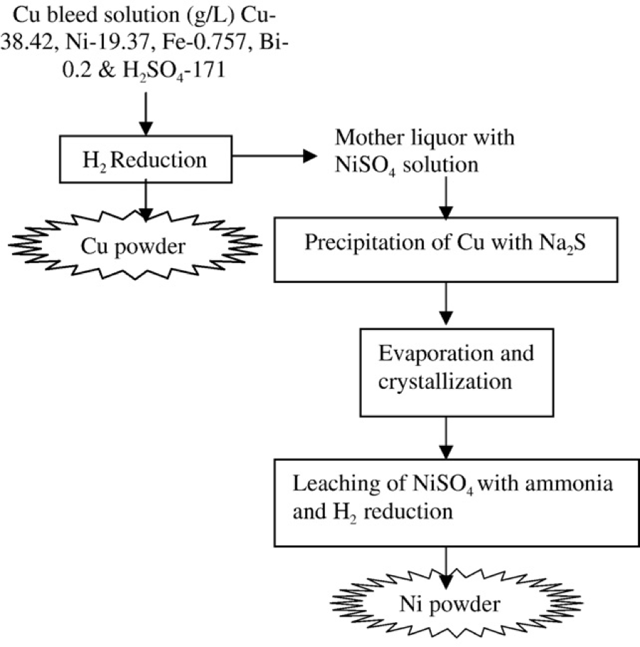
Fig. 6: Flow diagram for the treatment of copper bleed stream by hydrogen reduction (A. Agrawal et al., 2008.)
Overall, I have only just scratched the surface when it comes to methods of nickel recovery. Since high-grade nickel ores are far and few between these days, fluid-bed roasting and chlorine-hydrogen reduction of nickel matte are becoming less popular techniques. The recovery of nickel from low-grade ores is increasingly more important, from new techniques of pressure oxidation of the concentrate in an autoclave to bioleaching of the nickel in the ore, it is evident that time and money are being put into researching these processes. Solvent extraction and ion exchange are also used in the recovery of nickel from various bleed streams from the electrorefining of other metals such as copper. The unique properties of nickel such as corrosion resistance, conductive and magnetic properties, and the capability for electromagnetic shielding, are what make nickel such a valuable resource. In the future, more recovery and recycling of nickel from secondary sources and low-grade ores will be even more important as ore grades decline and primary resources are depleted.
References
Agrawal, A., Bagchi, D., Kumari, S., Kumar, V. and Pandey, B. (2008). Hydrogen reduction of
Agrawal, A., Kumari, S., Parveen, M. and Sahu, K. (2012). Exploitation of Copper Bleed Stream for the Extraction and Recovery of Copper and Nickel by Bis(2,4,4-
Agrawal, A., Manoj, M., Kumari, S., Bagchi, D., Kumar, V. and Pandey, B. (2008). Extractive separation of copper and nickel from
Anon, (2010). Mineral Commodity Summaries: Nickel. [online] Available at: https://minerals.usgs.gov/minerals/pubs/commodity/nickel/mcs-2010-nicke.pdf [Accessed 31 July 2017].
Anon, (2012). Mineral Commodity Summaries: Nickel. [online] Available at: https://minerals.usgs.gov/minerals/pubs/commodity/nickel/mcs-2012-nicke.pdf[ Accessed 31 July 2017].
Ambatovy.com. (2014). Ambatovy | Overview. [online] Available at: http://www.ambatovy.com/docs/?p=373 [Accessed 31 Aug. 2017].
Ashcroft, G. (2017). Nickel Laterites: The World’s Largest Source of Nickel. [online] Geology for Investors | Make sense of mining company investments. Available at: https://www.geologyforinvestors.com/nickel-laterites/ [Accessed 31 Aug. 2017]
Cheremisinoff, N. (2007). Handbook of solid waste management and waste minimization technologies. Norwich, NY: Knovel.
Cornwall, H. (1966). Nickel Deposits of North America. [ebook] Washington: United States Government Printing Office. Available at: https://pubs.usgs.gov/bul/1223/report.pdf [Accessed 31 July 2017].
Crundwell, F, Moats, M, Ramachandran, V, Robinson, T, & Davenport, WG 2011, Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals, Elsevier Science, Oxford. Available from: ProQuest Ebook Central. [31 July 2017].
Dalvi, A., Bacon,
Encyclopedia Britannica. (2017). laterite | geology. [online] Available at: https://www.britannica.com/science/laterite [Accessed 31 July 2017].
Ikotun, B., Adams, F. and Ikotun, A. (2016). Application of three xanthates collectors on the recovery of nickel and pentlandite in a low-grade nickel sulfide ore using optimum flotation parameters. Particulate Science and Technology, 35(4), pp.462-471.
Jones, J. (2017). Nickel Powders from the Carbonyl Process. [online] AZoM.com. Available at: https://www.azom.com/article.aspx?ArticleID=499 [Accessed 31 July 2017].
Kumar, V., Sahu, S. and Pandey, B. (2010). Prospects for solvent extraction processes in the Indian context for the recovery of base metals. A review. Hydrometallurgy, 103(1-4), pp.45-53.
Natural Environment Research Council (2008). Nickel Mineral Profile. Keyworth, Nottingham, UK: British Geological Survey, pp.1-9. Available at: http://www.bgs.ac.uk/mineralsUK/statistics/mineralProfiles.html
Nickel Institute. (n.d.). Where & Why Nickel is Used. [online] Available at: https://www.nickelinstitute.org/NickelUseInSociety/AboutNickel/WhereWhyNickelIsUsed.aspx [Accessed 31 Aug. 2017].
Schlesinger, M. and Biswas, A. (2011). Extractive metallurgy of copper. Kidlington, Oxford, U.K.: Elsevier.
Sen, P. (2015). T.T. Chen Honorary Symposium on Hydrometallurgy, Electrometallurgy, and Material Characterization, edited by
Wang, E. (2016). China imports of Philippine laterite ore hit in







