Electrowinning is a widely used technology in modern metal recovery, mining, refining, and wastewater treatment applications. The electrowinning process is one of the oldest electrolytic processes known and was first introduced in 1807 by English chemist Humphry Davy. After 66 long
“Modern technology Owes ecology An apology.”
-Wendell Berry
But before we dive deeper into understanding electrowinning let us look more generally at what an electrolytic process is, since electrowinning is an electrolytic process.
An electrolytic process is based on the principles of electrolysis.
To download fill the form below
In electrowinning process, the electrolyte includes dissolved metals that have to be recovered. Another similar process is electrorefining which is strictly used in refining applications to improve the purity of the metals. Both processes use electroplating and are used to purify non-ferrous metals such as copper and silver.
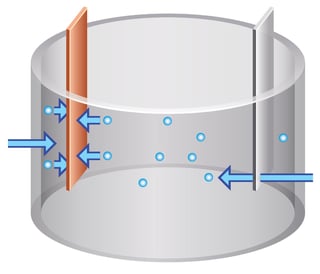
A conventional electrowinning unit consists of a tank, rectifier, and pump. Cathodes and anodes are aligned inside the tank. The pump fills the tank with an electrolytic solution. Electrical current is provided by electrowinning rectifier to cathodes and anodes in such a way that the difference in electrical potential creates a movement of cations towards the cathode. As time goes by the positively charged ions will plate on the cathodes. It is important to note that as metal builds up on the cathode the deposition of metal in the solution will decrease and the plating will slow down. Once the metal deposition decreases to a rate that is not sufficient for electroplating, the cathodes with pure metal deposited will be harvested. In the case of wastewater treatment, the solution (wastewater) will be cleaned or significantly purified of non-ferrous metals and can be further treated by chemical precipitation or reused within the industrial process.
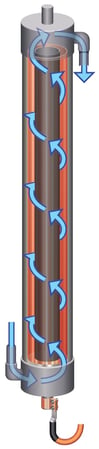
Electrowinning was mostly unchanged until 20 years ago with the advent of advanced electrowinning technologies that use cylindrical cells with high flow rates to enhance the rate of mass transfer, overcoming issues associated with depletion zones and enabling the production of high purity metals even in presence of impurities. Electrometals was the very first to develop and commercialize the first cylindrical electrowinning cell known as emew, which stands for Electrometals electrowinning. The advent of cylindrical electrowinning cells has expanded the applicability of electrowinning beyond just metal refining to recycling, waste and wastewater treatment, and even into hi-tech industries like semiconductors.
While electrowinning process is mostly used to recover non-ferrous metals such as copper, nickel, tin, and cadmium, or precious metals like gold, silver, and platinum, it also has a usage in industries that require wastewater treatment. An electrowinning facility can operate 24 hours a day, 7 days a week, and offers some great benefits:
- Reduction in waste and water use
- Enhanced process control
- Compliance with environmental permits
- Recovered metals sold for profit
- Stable recognized technology
Given all this electrowinning technology remains a core process in mining, refining, and metal finishing industries providing great financial and environmental benefits.
Sources:
https://en.wikipedia.org/wiki/Electrowinning
https://health.howstuffworks.com/wellness/diet-fitness/information/question565.htm
https://en.wikipedia.org/wiki/Electrolyte
https://en.wikipedia.org/wiki/Balbach_Smelting_%26_Refining_Company
http://www.nmfrc.org/pdfs2/metal-ewin.pdf






