Silver Recovery from Scrap and Low-Grade Residue
Topics: metal recycling, electrowinning, emew, Refining, metal powders
The electrowinning of copper is an electrolytic process that uses electricity to recover dissolved copper from solution as
Cu2+(aq) + 2e- -->Cu(s) (E0 = +0.34V)
Copper easily dissolves in acids including sulphuric, nitric and hydrochloric. Recovering copper cathode from acidic
Topics: copper, electrowinning, emew, Refinery Optimization
Topics: electrowinning, mining, emew, Refining, Refinery Optimization
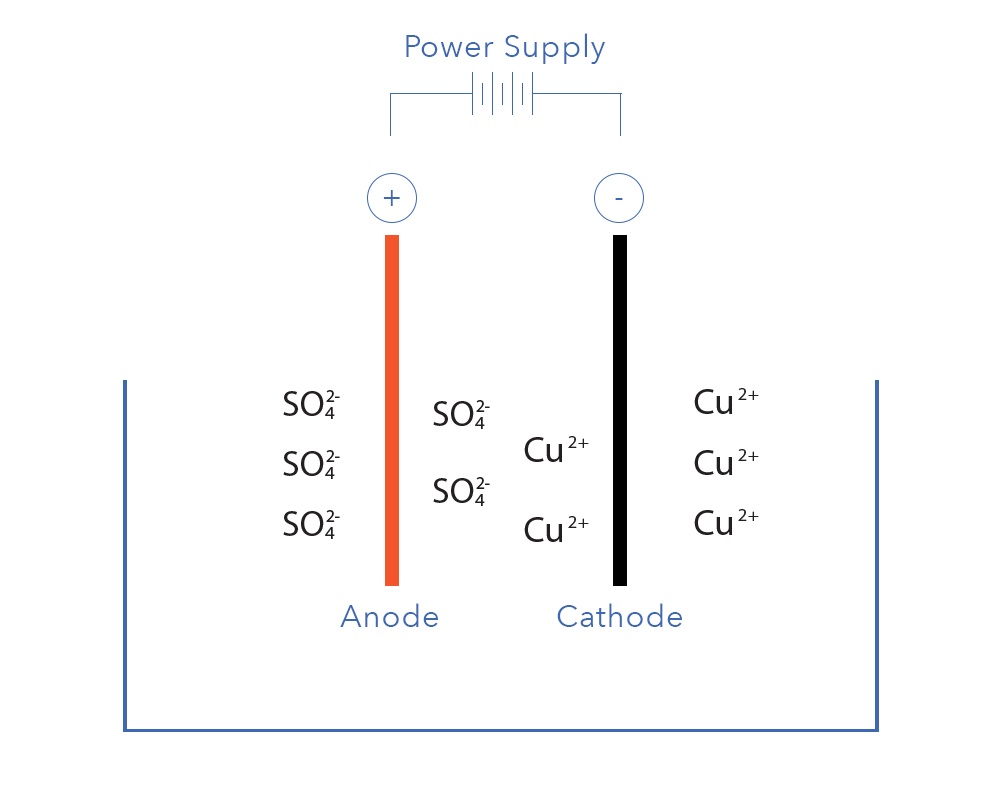
Electrowinning is known as an electrolytic process because it involves electrodes submerged into an electrolyte.An electrolyte is simply a conductive solution formed by dissolving positively and negatively charged ions.
Topics: electrowinning, emew, Refinery Optimization
Electrowinning is the process of ‘winning’ dissolved metals from solution by passing an electrical current through an electrolyte containing said metal. The fundamentals of the electrowinning process have been discussed in a previous blog. The relative ease of recovery depends on the electrochemical potential of the target metal relative to the Standard Hydrogen Electrode (SHE) which is defined by the following reaction:
Topics: electrowinning, nickel, emew, Refinery Optimization
Lab tests such as this are an ideal way to demonstrate the recovery capabilities of emew for a variety of electrolytes and feed materials. In most cases, these tests can be done right at your site so you can see the results for yourself.
This particular test is to demonstrate the recovery of copper cathode from a copper sulphate feed solution. We can test a range of feed materials to recover not only copper, but also silver, nickel, tin and others. This copper sulphate solution was prepared ahead of time and roughly 3L transferred to the feed tank.
Topics: copper, electrowinning, emew, General