Many industries generate acid mist as a result of their industrial processes. In most cases the acid mist will be caused by usage of inorganic acids, and in particular sulfuric acid. Sulfuric acid is mostly used in a production of fertilizers, and industries like pulp and paper, iron and steel, mining, refining and wastewater treatment. Acid mist is formed as a condensation of acid vapour and the extent of the mist will depend on the industry, process and solution itself.
"Pollution is a serious one. Water pollution, air pollution, and then solid hazardous waste pollution. And then beyond that, we also have the resources issue. Not just water resources but other natural resources, the mining resources being consumed, and the destruction of our ecosystem."
- Ma Jun
What is Acid Mist?
Acid mist is classified as carcinogenic to humans and proven to cause laryngeal and lung cancer in addition to tooth erosions and respiratory irritation. The most common exposure is through inhalation or dermal contact. In December 2009 European Union legislated a directive establishing a list of indicative occupational exposure limit values and acid mist was included in the directive. According to the document the limit value for sulfuric acid exposure is 0,05 mg/m3 over 8 hours. The commission was assisted by Scientific Committee for Occupational Exposure Limits to Chemical Agents (SCOEL). Many European countries adopted a strategy that is striving to reduce environmental risks and eliminate carcinogens from industrial uses. Many European laws and directives are aimed to eliminate or substitute processes that produce hazardous materials and point a threat to employees or environment.
A common source of acid mist is electrorefining and electrowinning used to produce high purity metals. Most conventional electrolysis plants used for metal recovery are what are considered “open” systems. A large bath of electrolyte with alternating planar anodes and cathodes across which a direct current is applied. During the electrolysis process, gases such as hydrogen and oxygen are generated at the anode. As these gases rise to the surface of the electrolyte, they burst resulting in acid mist. The result is a toxic and harmful environment for employees and visitors. In addition, acid mist is extremely corrosive and significantly reduces the functional life of all the factory parts that are exposed to it. According to a National Occupational Hazard Survey, a nationwide observational survey conducted in a sample of nearly 5,000 establishments in America, close to 500,000 workers are exposed to sulfuric acid. In order to comply with occupational exposure regulations, industrial processes have to be continuously reviewed. New strategies are being utilized by governments, industry and researchers to reduce or eliminate the risk of exposure to carcinogenic acid mist. Many companies are moving towards lean and green manufacturing that ensures a safe environment for workers while minimizing the environmental impact of their operations.
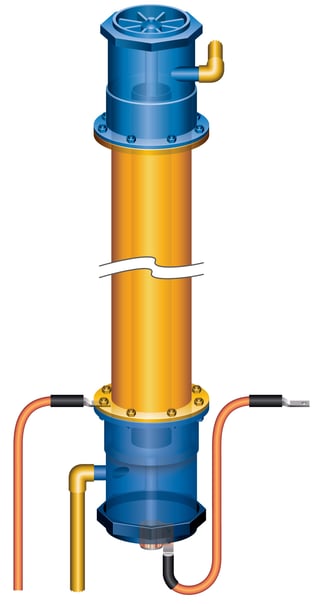
Different from conventional electrowinning plants, emew electrowinning technology offers a safe, efficient, patented technology that solves the problem of acid mist. In emew systems the electrowinning process is conducted in completely enclosed cylindrical cells. The gases evolved in the electrowinning process are flushed from the cells by the upward flow of electrolyte and back to the feed tank that is equipped with a mist eliminator. This approach completely eliminates acid mist for enhanced health, safety and environmental benefits.
What Additional Benefits Does emew Offer in Addition to Acid Mist Removal?
Are you looking for more direct and versatile options for metal recovery and wastewater treatment? Due to its inherent simplicity and efficiency, the emew electrowinning cell enables:
- Metal recovery in low metal concentration streams
- Higher productivity than conventional electrowinning cells
- Efficiency over a wider range of metal concentrations
- Higher tolerance to contaminants in solution
- Capability of electrowinning a variety of metals
- Higher purity products (99.99%)
- Electrowinning of either metal cathode or electrolytic metal powder
Q1: What happens to the gases that are produced as part of the emew electrowinning process?
To download fill the form below





