A review of why new technologies are going to drive the future of cobalt recovery and production.
Background of Cobalt:
Cobalt, the element that is just starting to create a lot of
Centuries ago, prior to the development of batteries, cobalt had other primary uses in construction, paints, and medicines. Cobalt can be magnetized, has high-temperature strength, hardness, and resistance to corrosion. It is these properties that make cobalt such a popular metal to use in alloys with aluminum and
Cobalt deposits are found naturally, with the largest known deposits in the Democratic Republic of the Congo (DRC), Canada, Australia, Zambia, and Brazil. The crustal abundance of cobalt is 26.6 ppm, falling significantly below the relative abundances of nickel, zinc, and copper in the Earth.
With a current recycling rate of cobalt of less than 30%, it poses a supply risk rating of 7.6/10. Cobalt forecasts suggest a rapid increase in demand in the coming years, especially with the push for electric vehicles. It is evident that recycling of cobalt, and cobalt recovery in general, are about to become hot topics, and important areas of interest for miners, producers, and primary users of cobalt.
Current Mining Methods for Cobalt Production:
Cobalt is found primarily with mineral deposits of nickel and copper in the Earth. There are many different cobalt ores that are mined, involving many steps of separation and purification. The most well-known and mined ores
Cobalt arsenides are processed using a roasting stage, followed by a pressure leach. Due to health and environmental
Copper-cobalt (oxides and sulfides) have a more complex series of steps that are followed to purify cobalt. Copper-cobalt ores are primarily found in Zambia and the DRC, where the cobalt is recovered from copper flotation concentrates, using a roast-leach-
Nickel sulfide deposits are processed using a variety of methods to produce both nickel and cobalt products. One very well-known process involves an ammonia leach, otherwise known as the Sherritt-Gordon process, using hydrogen reduction. The modified Sherritt-Gordon process is a pressure oxidation leach. Other methods include a sulfate oxidative leach, a chlorine leach – followed by chloride electrowinning, electro-refining of nickel matte anodes, and electro-refining of impure metal.
Nickel laterites (oxides) are processed using Caron or HPAL hydrometallurgical methods, because these methods allow for the recovery of cobalt, whereas pyrometallurgical processing will not.
A generalized process for cobalt recovery may include the following steps:
Step 1) Impurity Removal
- Impurities are precipitated with “lime” (CaCO3 – limestone, or Ca(OH)2 – milk of lime)
Step 2) Solvent Extraction (SX)
- Removal of copper, zinc, manganese, and cobalt-nickel separation
Step 3) Ion Exchange (IX)
- Removal/polishing of zinc, copper, and nickel
Step 4) Final Purification (Cobalt Recovery)
- Re-leach and electrowinning cobalt
- Can
electrowin in tandem with SX and IX such as SX-emew or IX-emew systems
Cobalt oxide was the main form of cobalt that was desired for many years, as it was used as a blue
Step-by-step, we can look at the different methods used to recover cobalt, of which there are many. Purification and recovery of cobalt can prove to be very difficult. Cobalt and nickel are found together not only in the Earth, but they are also found side-by-side on the periodic table. They have similarities in their aqueous chemistry that make them very difficult to separate. Both cobalt and nickel are divalent,
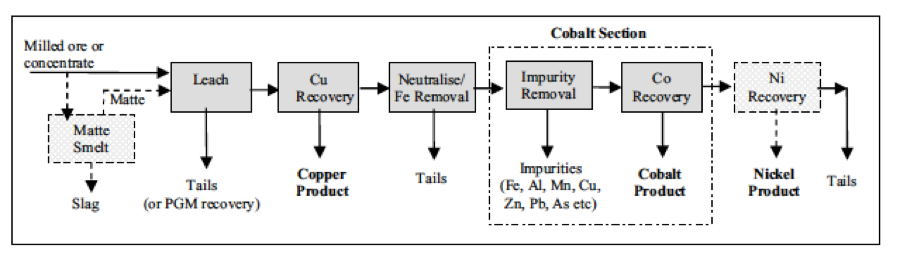
Fig. 1: Generalized Flow Sheet of Copper/Cobalt/Nickel Recovery (Fisher, 2011)
When designing processes for cobalt recovery, it is important to analyze and overcome some of the technical challenges that lie ahead. In terms of impurities, manganese is the most troublesome impurity for cobalt recovery, specifically from copper-cobalt ores, in which Mn is present in high quantities. Air/SO2 or O2/SO2 mixtures are used to oxidize and precipitate manganese from sulfates, commercially adopted at DRC copper-cobalt plants (Tenke, Fungurume, and Ruashi). The air/SO2 method has least disadvantages, especially at the larger scale.
Many improvements to cobalt processing have been implemented, in order to alleviate some of the more difficult challenges with cobalt recovery; these include, but are not limited to:
- Bio-leaching
- Heap bio-leach
- Bio-leaching for cobaltiferous pyrates
- Cobalt recovery from copper smelter slag
- Crystallization for cobalt sulfates
- Evaporation/crystallization can give high value if good grade, but it is dependent on Mn and Mg removal
- Problems with this method include its complexity, potential for gypsum contamination and scaling, and high energy costs
- Benefit: no reagents are required
- High temperature/pressure leaches for cobalt arsenides
- HPAL for nickel laterites
- Favoured over the Caron process or pyrometallurgical methods, HPAL has advanced to use larger autoclaves for higher
throughputs
- Favoured over the Caron process or pyrometallurgical methods, HPAL has advanced to use larger autoclaves for higher
- Hydrogen reduction for briquettes
- Cobalt powder is produced by hydrogen reduction in an autoclave
- Electrowinning is usually
preferred to this method, for lower capital and operating costs, and higher metal recovery
- Molecular recognition technology (MRT)
- MRT is similar to
IX, but uses a special resin that can target specific molecules - Can use this method in tandem with electrowinning, such as a MRT-
emew system
- MRT is similar to
- Precipitation of cobalt as an intermediate product
- A variety of reagents can be used to obtain different intermediate products
- NaHS (sodium hydrosulfide) or H2S to produce cobalt sulfide
- Ca(OH)2 for low-grade hydroxide/basic sulfate containing gypsum
Na 2 CO 3 , produces high-grade cobalt carbonateNaOH, produces high-grade cobalt hydroxide- MgO (magnesia), produces high-grade cobalt hydroxide
- All of these, with the exception of cobalt sulfide, are excellent feeds for electrowinning cobalt
- The sulfide route is a common choice in the nickel industry because it rejects manganese,
however it is not as common for cobalt as the process is complex and the product less saleable - Magnesia route is specialized, applied to mixed nickel-cobalt hydroxide (MHP) production in Australian laterite projects
- Benefits to the magnesia route:
- Cleaner, lower mass product
- Easy materials
- Hydroxide cake is easily filtered and re-leached
- Some Mn rejection
- MgSO4, Mg can be
precipitate with lime
- Problems with the magnesia route
- Large quantities of high-grade MgO
- Imported from
USA or Australia - Reagent is vulnerable to hydration and aging
- CaO in the MgO forms gypsum in the product
- Grades of MgO vary in effectiveness
- Benefits to the magnesia route:
- A variety of reagents can be used to obtain different intermediate products
- Resin-in-pulp (RIP)
- Resin is used to load target metals from a slurry, extracting cobalt from low-concentration, impure aqueous stream – transferring to high-concentration, high-purity solution for cobalt electrowinning
- Cobalt intermediate precipitation and re-leach processes are not required here, and manganese and magnesium are rejected
- Can neutralize the pulp directly with lime
- Strong oxidants for hydroxy-oxides
- Aimed to oxidize and precipitate cobalt preferentially over nickel
- Strong oxidants like ozone, Caro’s acid, or chlorine are used, making it very specialized and requires high safety management
- SX is usually
preferred to this method, due to poor cobalt/nickel selectivity
- Whole
ore leaching (WOL)- Applied to copper oxide ores, using additional acid to achieve improved recoveries in comparison to oxide ore flotation and concentrate leach
Variation within the processing steps and methods are based on the type of ore that is extracted, and the form of cobalt desired (ie. cobalt sulfide, or mixed Ni-Co sulfide precipitate (MSP); cobalt hydroxide, or mixed Ni-Co hydroxide (MHP); cobalt carbonate; cobalt oxide or oxy-hydroxide; cobalt sulfate; cobalt cathode; or cobalt powder). Each of these different forms of cobalt, metallic or compound, have different applications and end-uses, which is why there are many different methods for cobalt recovery, from various types of cobalt-containing ores.

End Uses of Cobalt:
Although cobalt was first used over 2000 years ago, new uses for this metal continue to put pressure on supply. Rechargeable batteries and electronics are the areas where cobalt is receiving the most attention, and recycling of cobalt from these devices is about to become a very important area of focus. Cobalt is used in electronics, either in their batteries, integrated circuits, and/or semiconductors. Rechargeable batteries contain cobalt oxide, cobalt hydroxide, or cobalt metal. Currently, the CDI reports that 50% of cobalt produced globally is found in rechargeable batteries; because of this, both the EU and USA classify cobalt as a critical raw material. The most commonly used type of battery at present is the lithium-ion battery, where cobalt is found in the cathode. Cobalt is also an important component of nickel-cadmium (NiCd) and nickel-metal hydride (NiMH) batteries. These batteries exist in portable devices, such as phones, laptops, tablets, and cutting tools; in EVs; and in stationary batteries, such as renewable energy power stations and home storage.

When thinking of the sustainable future of our planet, a lot of us think about renewable energy sources, but cobalt also plays an important role in making our current energy sources a little greener. Cobalt acts as a catalyst for desulfurization of natural gas and refined petroleum products. Just one ton of cobalt, when applied as a catalyst mixture, can reduce sulfur oxide (SOx) and nitrogen oxide (NOx) emissions by 25 000 tons and 750 tons, respectively. Cobalt is also used as a catalyst in the production of recyclable plastics.
Older, more traditional uses of cobalt are in alloys and inks and pigments. Cobalt plays a major role in alloys used for aerospace, prosthetics, automobiles, and industrial equipment. Cobalt oxide is characteristic to giving glass, porcelain, ceramics, paints, and inks a very bright blue
With so many uses of cobalt, ranging from new technologies to established industrial applications, cobalt reserves continue to be depleted. The DRC holds 50% of the world’s known cobalt reserves, and this supply won’t last forever. Many countries have been investing in new methods to supply their metals requirements, including alternatives to traditional mining and refining techniques. Recyclability of cobalt is a very important topic of discussion, as is
Maximizing Recovery – Electrowinning Cobalt:
As mentioned above, battery technologies are receiving more demand and attention, resulting in the need for cobalt metal increasing significantly over the next decade and beyond. It has been reported, by Bloomberg New Energy Finance, that the cobalt demand is expected to rise from 1 918 tonnes in 2016 to 90 579 tonnes by 2030. The supply and demand imbalance is reflected in the price of cobalt, which has more than doubled in price from where it sat at this time in 2015, climbing from ~ 25 USD/kg in September 2015 to ~ 60 USD/kg in September 2017.
One important question is how these future demands will be met by suppliers? Only so much cobalt can be extracted per year at each mine, and there are only so many natural reserves, which are readily being exploited. Environmental sustainability and responsibility are key issues as with any resource development project. This leaves cobalt producers to look at alternative methods for production of cobalt, notably cobalt recycling and cobalt recovery from complex, polymetallic secondary sources. Although cobalt can be a pesky metal to
The production of large quantities of cobalt cathode has posed many challenges to those who
Traditional cobalt electrowinning uses full sheet cathodes and undivided cells, but mines in Zambia and the DRC use blanks that don’t have edge strips, so the cobalt grows around the edges and cannot peel off the blank. There is
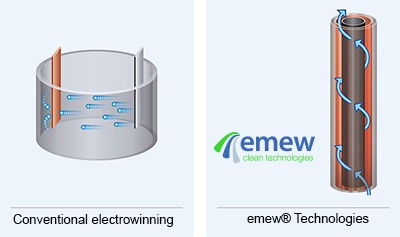
Division of cells can be carried out either by the use of anode bags, or cathode bags. For cobalt electrowinning, anode bags are commonly used;
The use of
Cobalt Recovery, what the Future Holds:
Cobalt is a metal that is contributing to a greener planet, as a catalyst for desulfurization of natural gas and refined petroleum products, and as a key component in rechargeable batteries, in both mobile and stationary devices. It’s important to analyze the life cycle of cobalt, how cobalt recovery can be maximized, and how to better promote and implement cobalt recycling. It seems counterproductive to extract cobalt in such an environmentally destructive way, only to integrate it into technologies and products that are considered “environmentally friendly”, or “sustainable”.

With such a promising outlook for so many sustainable technologies utilizing cobalt that impact our daily lives, it’s time for cobalt buyers, and suppliers, to think about how cobalt can be recovered in a more sustainable manner, and how innovative cobalt recycling methods can be implemented,
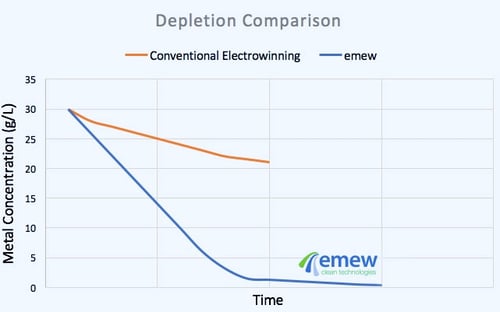
Recovery of cobalt and cobalt recycling are areas that need more focus, with attention to technologies such as electrowinning, reliable for extracting cobalt from low concentration waste streams, and scrap. Although cobalt electrowinning can be challenging, continued research and development of this technique for cobalt recovery is making it more practical even for small-scale operations. Maximizing recovery of cobalt from current cobalt extraction processes, as well as
References:
Cobaltinstitute.org. (2017). Cobalt Institute - formerly the Cobalt Development Institute (CDI). [online] Available at: https://www.cobaltinstitute.org [Accessed 22 Sep. 2017].
Fisher, K. (2011). Cobalt Processing Developments. Bateman Engineering Projects. Available at: https://www.saimm.co.za/Conferences/BM2011/237-Fisher.pdf [Accessed 18 Sep. 2017].
Ft.com. (2017). Funds switch on to battery commodities. [online] Available at: https://www.ft.com/content/ced7d86a-2fe9-11e7-9555-23ef563ecf9a [Accessed 18 Sep. 2017].
Infomine.com. (2017). Cobalt Prices and Cobalt Price Charts - InvestmentMine. [online] Available at: http://www.infomine.com/investment/metal-prices/cobalt/ [Accessed 18 Sep. 2017].
Mulaudzi, N. and Kotze, M. (2013). Direct cobalt electrowinning as an alternative to intermediate cobalt mixed hydroxide product. Mintek. Available at: http://www.basemetals.org/WhiteRiver2013/209-Mulaudzi.pdf [Accessed 18 Sep. 2017].
Periodictable.com. (2017). Abundance in Earth's Crust for all the elements in the Periodic Table. [online] Available at: http://periodictable.com/Properties/A/CrustAbundance.v.html [Accessed 22 Sep. 2017].
Rsc.org. (2017). Cobalt - Element information, properties and uses | Periodic Table. [online] Available at: http://www.rsc.org/periodic-table/element/27/cobalt [Accessed 22 Sep. 2017].








