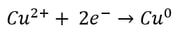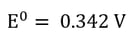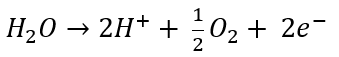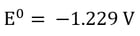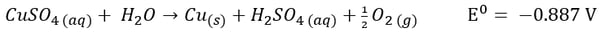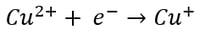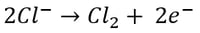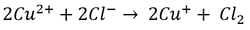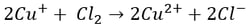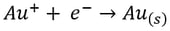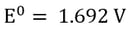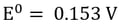A unique feature of the electrowinning process is acid generation. As a result, acid can be recycled or recovered from the metal depleted electrolyte. In particular, high concentrations of acid can be produced in an emew cell due to their unique ability to deplete to very low concentrations of metals (< 1 g/L).
This is most easily described by showing the electrochemical reactions occurring in an electrowinning cell during copper plating. Recall, from our blog on the Electrochemical Series the less negative the electrochemical potential, the more likely the reaction is to occur.
| Reduction at the Cathode (Half-Reaction) |
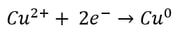 |
|
| Oxidation at the Anode (Half-Reaction) |
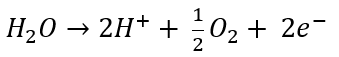 |
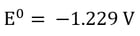 |
If the electrolyte is a copper sulfate solution, the overall reaction is as follows:
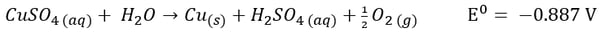
Here, it’s easy to see how acid is generated during electrowinning. Hydrogen ions are generated at the anode during electrowinning, the acid formed in the electrolyte will depend on the counter ions present. Sulfate-based solutions will generate sulfuric acid, whereas nitrate-based solutions will generate nitric acid. The chemistry in chloride based solutions, however, is slightly different due to the propensity of chloride to be oxidized at the anode producing chlorine gas. For example, in a solution of copper (II) and chloride ions, the redox reaction reactions are as follows:
|
Reduction at the Cathode (Half-Reaction)
|
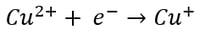 |
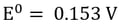
|
|
Oxidation at the Anode (Half-Reaction)
|
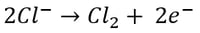 |
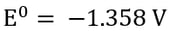
|
Once chlorine gas has been generated, it reacts with the newly generated copper (I) to re-oxidize the copper back to copper (II), generating a continuous cycle of Cu(II) to Cu(I) and back, without plating Cu(0).
|
Redox Reaction in the Cell
|
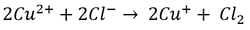 |
|
Reaction in Solution
|
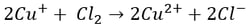 |
It’s important to note that the above reactions depend on the concentration of ions in solution (which determines Gibb’s free energy of the reaction) as well as reduction potential. If gold were in the same solution, there would be no problem reducing gold (I) to gold metal, because of gold’s very positive reduction potential, however chlorine gas would still be generated at the anode.
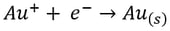
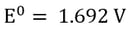
In some cases, acid generation can be an advantageous trait of electrowinning, however for metals like nickel that are pH sensitive, acid generation can be detrimental to the apparent efficiency of the reaction and the quality of the metal plated. There are methods for monitoring and adjusting pH during electrowinning to avoid this problem.
Acid generation in electrowinning can create a closed-loop process as it can be utilized in many ways within a process plant. Generated acid can be recycled back to the leach stage and used along with fresh acid for leaching of the selected metal; this will help minimize the volume (and cost) of fresh acid required. Another option for recycling is within a bleed stream; if electrowinning is used on a copper refinery bleed, the acid generated during electrowinning can be recovered using an Acid Purification Unit (APU) and re-used in the refinery, as opposed to topping up with fresh acid. APUs use ion exchange (IX) resins to adsorb strong acids and exclude metal salts. The resin beads can then be washed with water, liberating “clean” acid. The acid generated during electrowinning will be depleted of the particular metal of interest, but may still include some impurities, depending on the feed solution. By using an APU in tandem with electrowinning, it is possible to recover pure cathode and clean(er) acid.
It’s important to acknowledge the fact that acid generation in an electrowinning cell isn’t always positive. For example in silver electrowinning the silver can be re-dissolved into the electrolyte after it has been plated at very high concentrations of acid; this will reduce the apparent current efficiency of electrowinning. This can be easily overcome by making continual pH adjustments to the electrolyte during electrowinning or using an external acid recovery system similar to an APU described above.
The take-home message is that acid generation is inevitable during electrowinning in low pH solutions, but it can be a valuable part of the process. The acid generated will always depend on the electrolyte, specifically which ions are in the solution and their concentrations. By reusing or recycling this regenerated acid within an integrated process (with or without an APU), acid generation can go from being an undesirable side effect to a valuable by-product.