Silver refining is a critical part of any precious metals refinery. Often the higher value metals such as gold and the Platinum Group Metals (PGM’s) are associated with silver, which must be recovered separately as a by-product.
As with other refined metals, the selling price of the final product is dependent on the purity, therefore precious metals refiners are inherently obsessed with purity as well as inventory (aka working capital).
While LMBA Good Delivery Bars have
Silver is commonly
Pure silver is deposited onto the cathode as a powder, while gold is left behind on the anode as a slime. Pure silver powder is harvested from the cathode manually or using mechanical wipers that scrap the silver from the cathode, before being dewatered and washed.
To download fill the form below
The concentration of copper and other base metals that are corroded into solution continuously build up to a steady state concentration which is controlled by way of an electrolyte bleed.
The volume of bleed required depends on the concentration of impurities in the silver anode, and the predisposition of these metals to co-plate with silver thereby affecting the purity of the silver plated on the cathode.
The bleed is
The spent silver anodes are recycled to re-melt when the anodes have corroded to the point where they are almost falling apart (typically 48 hours).
The gold slimes recovered from the spent silver anodes are melted into impure gold anodes for further refining into pure gold, while the spent silver anodes (typically 15-20% of the original anode weight) are re-melted into fresh anodes to start the electrorefining process anew.
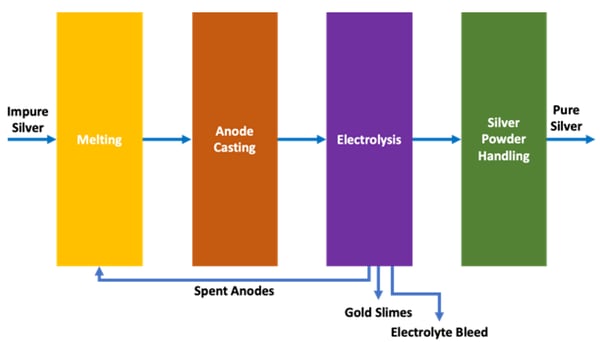
This method of conventional silver electrorefining has been used for many years, however there are several disadvantages that any silver refiner has to contend with:
- Silver purity is acutely affected by impurities in the feed material
- Silver is often manually harvested from the cathodes by operators
- Working capital is high due to the time (48h) required to recover gold from spent anodes, and
continuous recycle of spent silver anodes Requirement for an anode casting circuit to feed silver to the electrorefining circuit- Silver losses are common due to electrolyte bleed treatment, recycling of spent anodes and manual harvesting
Make up solution is required to replace the bleed electrolyte- Silver needs to be recovered from the bleed electrolyte – this typically requires messy and expensive chemical precipitation with chloride or cementation with copper.
A significant advancement to the silver refining process has been developed by
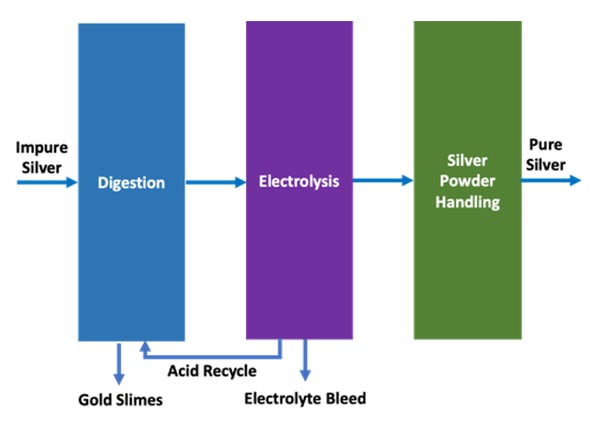
The silver-rich electrolyte is fed to the fully automated
The high flow of electrolyte across the cathode surface significantly improves mass transfer and enables high purity silver to be electrodeposited even in the presence of high concentrations of impurities such as copper.
The pure silver deposited on the cathode is automatically harvested by a reverse flush cycle and collected in a secure, conical bottom tank before dewatering and washing.
The
The advantages of the
- High purity silver (up to .99999)
- Lower inventory and working capital of silver and gold
- Simple, fully automated operation
- No requirement for anode casting and doré handling
- No spent anodes to recycle
- Smaller footprint than conventional electrorefining cells
- >99.99% of the silver is recovered without the need for cementation
- Wide operating range of silver and impurities
- Demonstrated ability to operate at 40% over design capacity
- No cementation required for the electrolyte bleed, and no cementation cake to reprocess
Fully enclosed and automated system requiring lesslabour and resulting in fewer losses
The investment to upgrade an existing precious metals refinery from conventional electrorefining to
A precious metals refinery treats 500 kilograms per day of silver (5 million oz/a) using conventional Moebius electrorefining cells.
The existing cells are nearing the end of their usable life, and the refinery manager is deciding between upgrading to a new electrorefining
The current process using Moebius cells has a turnaround time of 48-72 hours from the time the silver (and gold) enters the
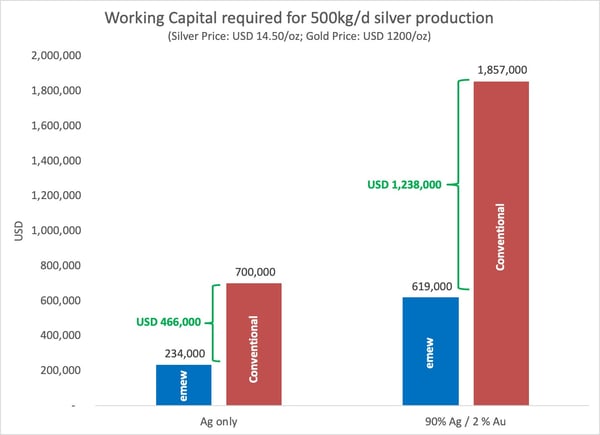
Furthermore, the inventory required to produce 500 kg/d of pure silver is three times this amount, that is to
Due to the presence of copper in the feed material, the current operation struggles to achieve .9995 silver purity so one of the requirements of the new system is to be able to consistently achieve .9999 silver.
The existing anode casting circuit and silver harvesting
Metallurgical accounting at the end of each month is difficult due to the high working capital, losses due to manual silver harvesting, not to mention the handling of brittle spent anodes and the anode bags of gold and PGM slimes.
The refinery manager is sold on the benefits of
The capital expenditure for an
Assuming
Whilst it is true that the cost of this working capital or inventory can be financed it is also true that plant and equipment can be financed, and customers are always looking for faster returns on their gold and silver.
For every additional 1% gold in the doré feed material, the additional working capital is equal in value to the
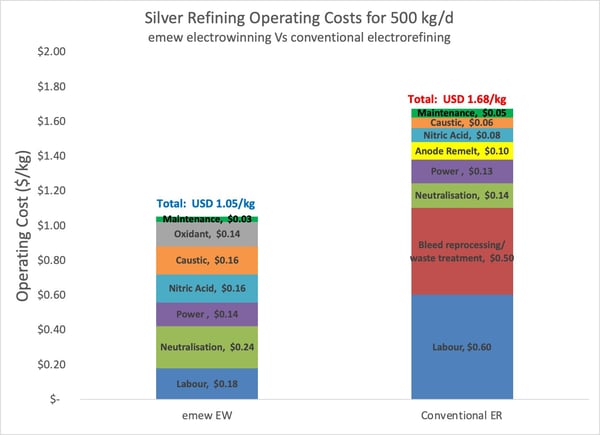
Now let’s take a look at the breakdown of operating costs of
Overall, the operating costs for
The main operating cost of conventional electrorefining is
Anode handling represents 6% of the total operating cost with conventional electrorefining – a cost that is not required with
Melanie presented the following case to her management to include in the next year capital budget:
| Capital requirement for 5m oz/yr refinery (installed cost) | $800,000 |
| Expected working capital savings (minimum 1 day) | ($466,000) |
| Savings on repairs required to existing system | ($120,000) |
| Net capital requirement | $214,000 |
| Operating cost savings @$0.63/kg | $110,250 |
| Payback period | < 2 years |
In addition to the financial benefit above the marketing department has indicated that production of 1 million ounces per year of .99999 silver could command a premium of $0.15/oz adding an additional $150,000/yr of revenue.
While this is a bit speculative and needs some market testing, the markets requiring high purity silver are growing and include semiconductor, chemical vapor deposition (CVD), physical vapor deposition (PVD) display and optical applications.
In summary, the operating costs of emew are 37% less than conventional silver electrorefining, primarily due to lower
Furthermore,





