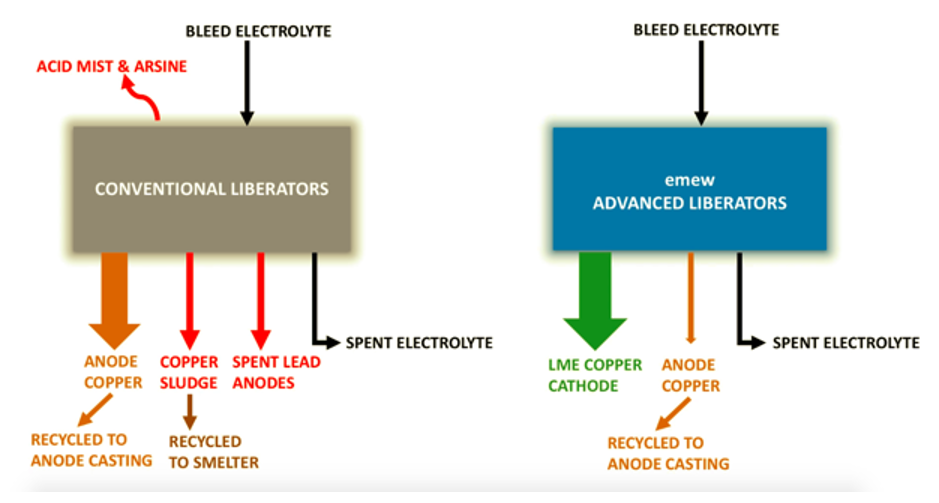
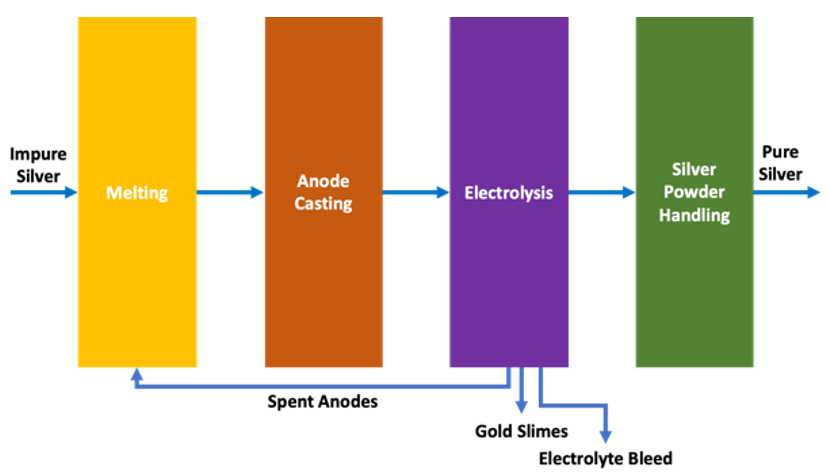
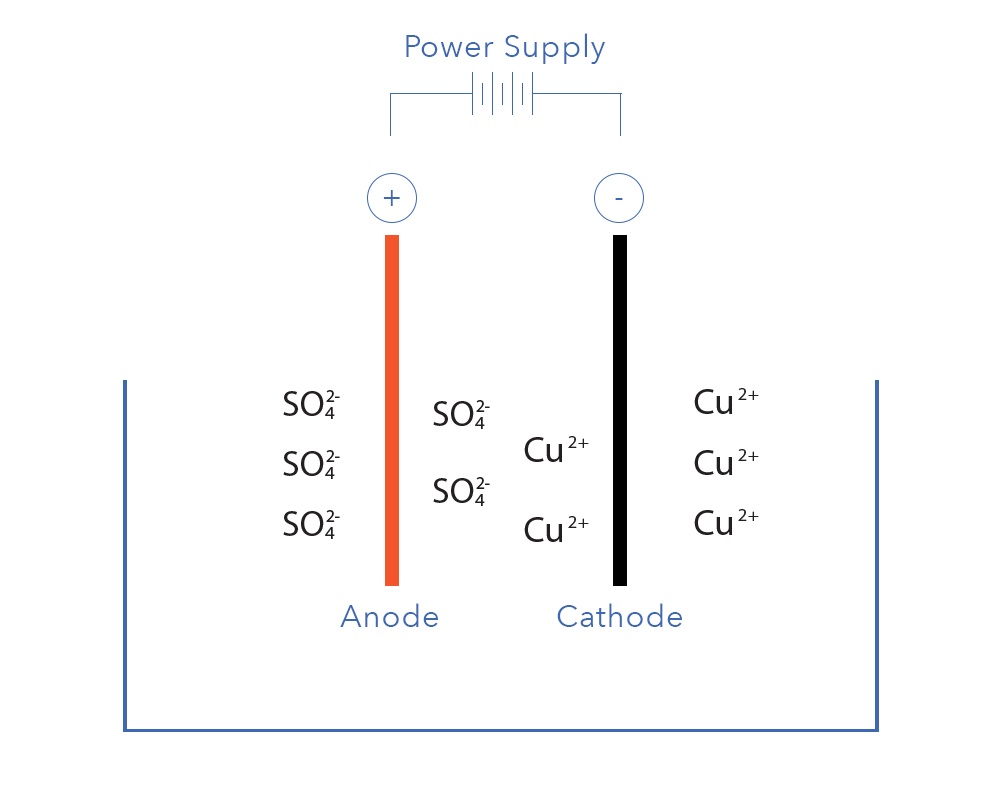
Electrowinning is a very convenient and robust way to directly recover dissolved metals such as copper, silver, gold, nickel, cobalt, and tin from their aqueous states in a particular electrolyte. Not surprising then when talking about metal recovery that we tend to focus most of our attention on those metals that we are interested in with the aim of recovering them from solution. That is after all the objective of the metal recovery process. What is less often talked about however are the impurities that can affect metal recovery with electrowinning. This blog post will identify those impurities that are most detrimental and discuss their effect on the electrowinning of some commonly recovered metals from aqueous solutions.