Electrowinning is a very convenient and robust way to directly recover dissolved metals such as copper, silver, gold, nickel, cobalt, and tin from their aqueous states in a particular electrolyte. Not surprising then when talking about metal recovery that we tend to focus most of our attention on those metals that we are interested in with the aim of recovering them from solution. That is after all the objective of the metal recovery process. What is less often talked about however are the impurities that can affect metal recovery with electrowinning. This blog post will identify those impurities that are most detrimental and discuss their effect on the electrowinning of some commonly recovered metals from aqueous solutions.
To download fill the form below
Iron as Ferric (Fe3+) Ion
In the recovery of base metals such as copper, nickel, cobalt, and tin as well as precious metals such as gold and silver, iron can be a major concern and it can be overlooked when considering the process chemistry. In aqueous solutions, oxidized iron species can be present as ferrous (Fe2+) and/or ferric (Fe3+) ions. In an electrochemical cell, iron can be problematic owing to the ferrous/ferric redox couple.
At the anode, ferrous (Fe2+) ion is oxidized to ferric (Fe3+) ion by losing an electron (e-):
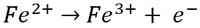
Meanwhile, at the cathode, ferric (Fe3+) ion is reduced to ferrous (Fe2+) ion by gaining an electron:
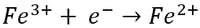
While these reactions seemingly balance each other out, they actually consume electricity and have a detrimental affect on current efficiency. Current efficiency is the actual amount of metal recovered divided by the theoretical amount that should have been recovered according to Faraday’s Law. The most significant operating expense associated with electrowinning is power consumption, therefore any reaction that competes with the primary metal recovery reaction is unwanted as it serves to reduce current efficiency.
In the case of copper electrowinning, the preferred reaction at the cathode is:
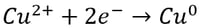
If there is a significant amount of ferric ion present in solution along with copper ion, the ferrous/ferric redox couple will consume electricity and decrease the efficiency of copper recovery. For this reason, as a rule of thumb we typically recommend a copper to ferric ratio of greater than six to ensure that the ferrous/ferric redox couple does not unwantedly consume electricity. If the copper to ferric ratio is less than six, an iron removal step such as precipitation, solvent extraction (SX) or ion exchange (IX) may be necessary.

Chloride
Another impurity that can be very problematic for recovery of metals using electrowinning from aqueous solutions is chloride. Chloride is a common impurity that can be found in relatively high concentrations in both solid and aqueous feed materials. The problem with chloride is twofold. On one hand, chloride itself is corrosive and will corrode wetted stainless-steel parts especially at concentrations exceeding 1 g/L. In electrowinning systems, including emew, the cathode is often composed of stainless-steel. In the presence of chloride, the stainless-steel cathodes and other stainless-steel wetted parts will begin to develop pin-holes and over time corrode to the point where they will need to be replaced. This decreases the lifetime of the equipment and contributes to increased maintenance and replacement costs. The other problem with chloride is that it is oxidized at the anode producing chlorine gas by the following reaction:
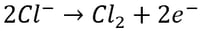
Chlorine gas is extremely toxic and corrosive therefore minimizing production of this gas within the electrowinning cells during metal recovery is very important.
As a rule of thumb, we typically recommend keeping chloride levels below 50ppm to minimize corrosion and chlorine production. At higher levels, corrosion resistant materials such as titanium may be used for wetted parts including the cathode.
Fluoride and Bromide
For recovery of metals from acidic electrolytes, electrowinning systems such as emew utilize oxygen evolving anodes comprised of a titanium substrate with a catalytic mixed metal oxide (MMO) coating. These MMO anodes are very robust but can be particularly susceptible to some impurities such as fluoride and bromide. As is the case with chloride forming chlorine gas, fluoride and bromide are also oxidized at the anode to form fluorine and bromine gas respectively. These gases are extremely corrosive and will effectively dissolve the titanium substrate leaving the MMO coating to simply disintegrate.
As a rule of thumb, we typically recommend keeping fluoride and bromide levels below 5ppm to ensure maximum longevity of the MMO anodes used in emew electrowinning cells.

Organic compounds
Certain organic compounds, such as thiourea, glues and surfactants can also be problematic for the MMO anode coatings as they may be oxidized at the anode and adhere to the coating. This can result in passivation and premature failure of the anode. Strong chelating agents such as EDTA can also be problematic as they can have a strong affinity for precious metal oxides present in the MMO coating. If EDTA binds with the precious metal oxides they can be stripped from the matrix and destroy the MMO coating.
If organics are present in the electrolyte it may be possible to remove prior to electrowinning with the use of activated carbon or similar pre-treatments. Unfortunately, these may not be effective for strong chelating agents such as EDTA hence it is important to understand the origin and chemistry of the electrolyte when designing a metal recovery process.
Summary
In general, metal recovery using electrowinning is a very robust process that can accommodate a wide range of concentrations, operating conditions and impurities. There are a handful of impurities that are notable exceptions and attention should be paid to these when evaluating the applicability of a metal recovery process for a particular electrolyte. Knowing the concentrations of ferric, chloride, fluoride and organic compounds will enable a metal recovery process to be tailored to a particular solution matrix and ensure the effective and economic recovery of a given metal of interest.
Have a question about your metal recovery process? Get in touch!





