Electrowinning – an introduction
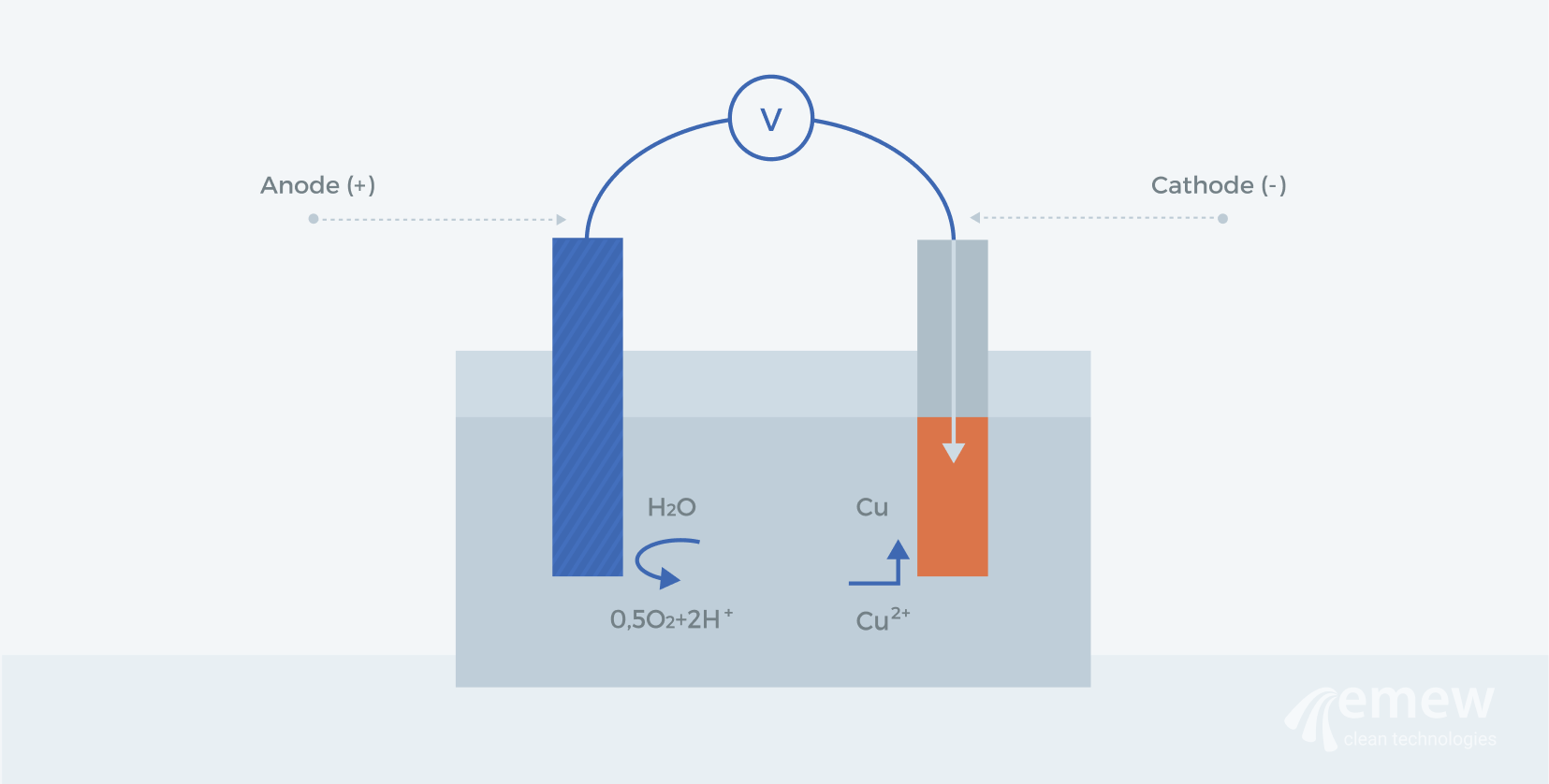
Electrowinning is a process used to recover metals from a solution or an electrolyte, by means of an electrolytic chemical reaction. This occurs when an electric current passes from a cathode that's negatively charged to a positively charged anode through a metal-containing solution. During this process, the electrons reduce the metal ions in the solution to form a solid metal deposition on the cathode. The quality of the metal produced can vary depending on the individual process and may require further processing or refining.
Metals that are recovered using electrowinning have typically been leached from the ore during an earlier processing stage, and examples include lead, copper, gold, silver, zinc, cobalt, and rare earth metals.
In the case of copper electrowinning, the copper ion in solution (Cu2+) at the cathode will react with two electrons (e-) to produce solid copper (Cu) as per the simplified equation below:
𝐶𝑢2+ + 2𝑒− → 𝐶𝑢
While copper is being reduced at the cathode, a simultaneous reaction will occur at the anode to oxidize water (H2O) to produce two hydrogen ions (H+), oxygen gas (O2), and two electrons, as shown in the simplified equation below:
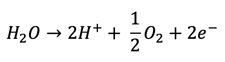
How impurities affect the electrowinning process
As the electrowinning process requires the transfer of electrons from the cathode to the anode through the electrolyte, anything that affects this transfer of electrons will influence the electrowinning process. Impurities in the electrolyte are a common cause of this interference and will typically affect the transfer of electrons in the following ways:
1) By coating the cathode or the anode (typically caused by organic impurities);
2) By damaging the electrolytic cell (typically caused by excessive chlorine, bromine, or fluoride); or
3) Through competing electrolytic chemical reactions (typically caused by excessive iron in solution).
Another major influence that impurities can have on the electrowinning process is in the deposition of the metal on the cathode. The two most notable effects on the deposition process are in the quality of the physical deposition (also known as the morphology), such as the formation of holes in the deposition plate or in chemical impurities in the deposition product reducing the final grade.
Other effects include altering the size, shape, or form of the deposition.
How to identify impurities in the electrolytic solution
While an ideal electrolyte would be a pure acid/metal salt-containing no other elements, this is not practically achievable, so impurities are always present.
Direct chemical analysis of the electrolyte is the best and most common way of determining which impurities are present. In an operational environment, this is usually completed using an online tool to allow for real-time analysis of the solution and to provide input into the daily operational decisions. Samples of the solution will be routinely taken and analyzed in a laboratory to ensure the accuracy of any automated analyzer and to provide calibration points.
It is also possible to identify impurities through observation of the electrowinning process. These indirect methods include:
- Electrolyte color
- Pitting or holes in the metal deposition
- Evidence of corrosion
- Beading or ridging of the metal deposition
- High power consumption
- Low metal recovery
Are impurities always harmful in electrowinning?
Many impurities will have a detrimental effect on the electrowinning process in very low concentrations, such as the presence of 0.02 parts per million (ppm) antimony when electrowinning zinc, but many impurities can be tolerated in much higher concentrations before having a significant effect on the process.
Further to this, impurities very rarely occur singularly, and there are often two or more impurities working in combination to affect the electrowinning process, and a combination of impurities is often more detrimental than the same elements singularly. For example, nickel and cobalt together will often be more detrimental to an electrowinning circuit than the presence of nickel or cobalt separately.
It is also possible that additives can be intentionally introduced to produce a more desirable final product. For example, organic additives can be used to control the morphology of the deposition of copper in an electrowinning circuit, affecting properties such as the grain size and the deposition texture.
In electrowinning cells that contain manganese as an impurity, it is also sometimes beneficial to have another impurity, iron, present to help counter the impact of the manganese. Iron can help stop the formation of permanganate, which can have detrimental effects on downstream processes.
The Ferric (Fe3+) – Ferrous (Fe2+) redox couple
One of the most common metallic elements found in any mineral deposit is iron, and it is, therefore, one of the most common impurities in any leachate or electrolytic solution. The iron can be present as ferric (Fe3+) ions or ferrous (Fe2+) ions depending on the mineralogy of the ore source, and in an electrowinning cell, it is possible to create a ferric-ferrous redox couple as described below.
At the cathode, ferric (Fe3+) ion is reduced to ferrous (Fe2+) ion by gaining an electron as described by the following equation:
𝐹𝑒3+ + 𝑒− → 𝐹𝑒2+
At the anode, ferrous (Fe2+) ion is oxidized to ferric (Fe3+) ion by losing an electron as described by the following equation:
𝐹𝑒2+ → 𝐹𝑒3+ + 𝑒−
With these two half-reactions occurring simultaneously during the electrowinning process, there are no net products created. However, an electrical current is consumed that would otherwise have been utilized to generate the target metal. This reduces the current efficiency, which is a measure of the total metal recovery compared to the theoretical metal recovery and reduces the overall efficiency of the electrowinning process.
With a reduced cell efficiency, the amount of power required to produce the final product increases. Given that the highest operating cost in an electrowinning cell is typically the power required, this ferric-ferrous redox reaction can drastically increase the total power consumed to generate the final product.
How much iron is too much in electrowinning?
Determining how much iron is detrimental to the current efficiency of the electrolytic cell is difficult as it depends on a number of factors. These include:
- Type of iron in ore (ferric or ferrous)
- Target metal (copper, zinc, lead, cobalt, etc.)
- Target metal purity
- Mine or operational strategy
- Other impurities present
- Concentration of metal ions in solution
A global survey of iron content in copper electrolytes reported that iron contents of less than 3 grams per liter (GPL) generally returned current efficiencies of greater than 90%, while iron contents ranging from 6 GPL to 9 GPL returned current efficiencies of 80% or less.
In general, an electrowinning cell will still operate at high current efficiencies with moderate levels of iron present in the electrolytic solution. As an example, in a typical copper recovery cell, a copper to ferric ratio of greater than ten will ensure the efficient recovery of copper without excessive electricity consumption.
The only way to reliably determine the effect that iron will have on an electrowinning circuit is to perform small-scale test work or to collect operational data.
How do you control the iron in an electrolytic solution?
The best way to control the level of iron in the electrolytic solution is to stop it from entering in the first place. Electrowinning is found at the final stages of the metal recovery process, and as such, there are many preceding stages where any unwanted iron content can be removed. Examples of these include:
1) Selective Mining
Effective mine planning will ensure that areas of high iron content are flagged and only economically viable ore is mined and processed further. Similarly, areas with very little iron can be flagged and combined with other ore types to create a ´blended´ or to provide more consistent feed.
2) Beneficiation
Treating the ore through a processing plant should selectively remove the unwanted iron using various techniques, including magnetic separation, density separation, flotation, and selective leaching.
3) Roasting
Treating the ore at high temperatures and with varying degrees of oxygen can allow the removal of iron-bearing minerals that could not have been removed in an untreated state.
The effectiveness of the options to control the amount of iron in the solution is dependent on the relative costs of each step and the unique nature of each operation. As an example, some operations have multiple ore bodies to choose from and can perform significant ore blending strategies, while others may have environmental restrictions that do not allow for a roasting stage to be performed. As such, it is not always viable to minimize the amount of iron in the electrolyte.
Can you remove or isolate the iron in an electrolytic solution?
If, after all prior processing stages, there is still a reduced current efficiency due to high iron content, it is possible to treat the solution to remove the iron, with two typical options being ion exchange and iron purification.
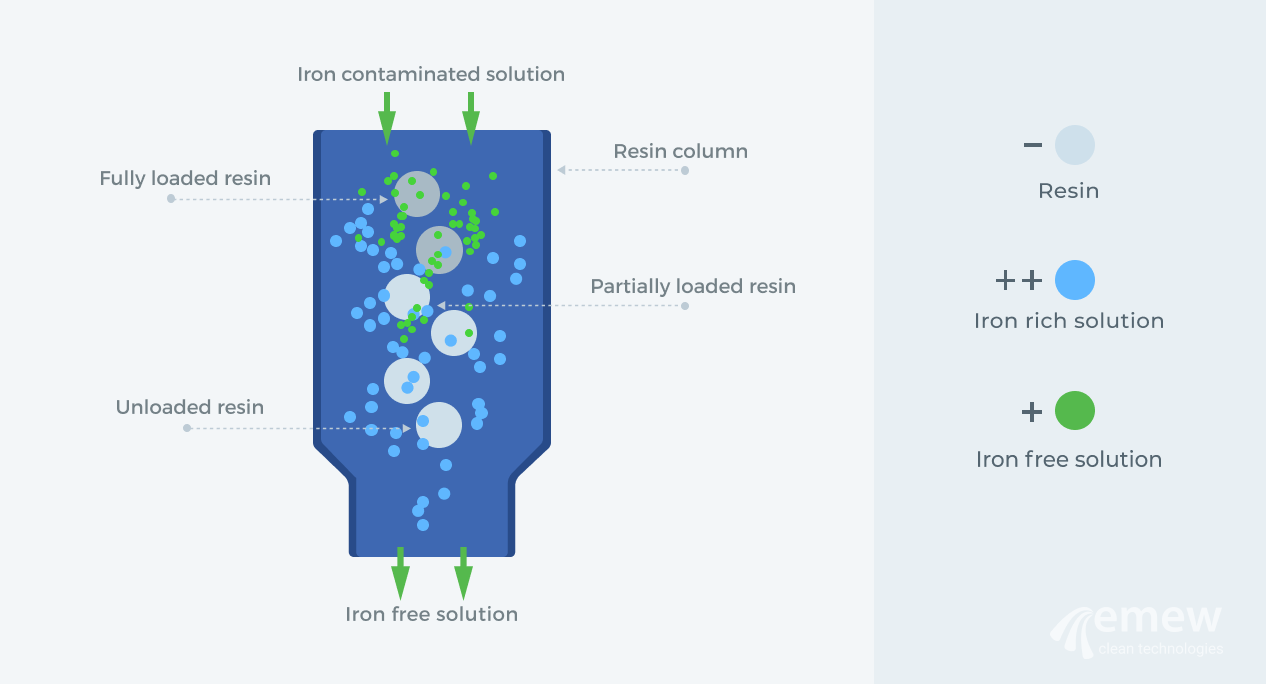
Iron exchange involves taking part or all of the electrolyte with the high iron content and passing it through a column containing a specially designed resin. The resin takes the form of thousands of small spheres packed into a column, and as the electrolyte is pumped through the column, the resin absorbs any dissolved iron and thus removes it from the electrolyte. The electrolyte can then be processed efficiently through the electrowinning cell. The resin, which is now ´loaded´ with iron, can be ´stripped´ of that iron with a strong acid, and then it is ready to be used again for another batch of high iron electrolytic solution.
Iron purification involves oxidizing any ferrous iron to ferric iron and then adding a neutralizing agent to precipitate any ferric iron as a hydroxide. This leaves a sludge containing most of the excess iron, which can be separated from the electrolytic solution that can then be processed in the electrowinning cells. Possible precipitation agents are limestone or zinc oxide, and this iron precipitation has the added advantage of precipitating some other undesirable impurities that may be otherwise difficult to remove, such as arsenic or germanium.
The viability of iron removal will depend on the operating strategies and the economics of each individual operation as these steps add significant costs to the initial outlay in capital expenditure and in continued operation expenses.
Summary
Minimizing the negative effects from impurities in an electrowinning circuit is imperative to ensure that the cell operates at high current efficiency while producing a good quality product. This is particularly important with regards to the presence of iron, as the formation of a ferric-ferrous redox reaction couple can absorb significant current and power away from the primary objective of the valuable metal.
While controlling the iron content from entering into the solution through effective mining and mineral processing is the best level of control, there are effective methods to remove the iron from the solution to ensure the electrowinning process is efficient.
Sources:
1. Fosnacht 1983, Effect of impurities on Zinc
https://www.researchgate.net/publication/225599911_The_effects_of_certain_impurities_and_their_interactions_on_zinc_electrowinning
2. Muresan 2000, Organic additives on copper electrowinning
https://www.researchgate.net/publication/248401861_Effect_of_some_organic_additives_upon_copper_electrowinning_from_sulphate_electrolytes
3. Subbaiah 1994, Effect of impurities on mass transfer during copper
https://ur.booksc.me/book/2872835/510492
4. Wever 1959, Effect and Control of Iron in Zn Electrowinning
https://aimehq.org/resources/digital-library
5. Evans, 2016 Significance of Electrometallurgy
https://www.researchgate.net/publication/301263182_Introduction_and_the_Significance_of_Electrometallurgy






